
JWH-147 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 11.0 nM at CB1 vs 7.1 nM at CB2. It was discovered and named after the renowned professor of organic chemistry John W. Huffman.

JWH-307 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 7.7 nM at CB1 vs 3.3 nM at CB2. It was discovered by, and named after, John W. Huffman. JWH-307 was detected as an ingredient in synthetic cannabis smoking blends in 2012, initially in Germany.

JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. It is one of the most potent 4-substituted naphthoyl derivatives in the naphthoylindole series, having a higher binding affinity (i.e. lower Ki) at CB1 than both its 4-methyl and 4-n-propyl homologues JWH-122 (CB1 Ki 0.69 nM) and JWH-182 (CB1 Ki 0.65 nM) respectively, and than the 4-methoxy compound JWH-081 (CB1 Ki 1.2 nM). It was discovered by and named after John W. Huffman.

JWH-019 is an analgesic chemical from the naphthoylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is the N-hexyl homolog of the more common synthetic cannabinoid compound JWH-018. Unlike the butyl homolog JWH-073, which is several times weaker than JWH-018, the hexyl homolog is only slightly less potent, although extending the chain one carbon longer to the heptyl homolog JWH-020 results in dramatic loss of activity. These results show that the optimum side chain length for CB1 binding in the naphthoylindole series is the five-carbon pentyl chain, shorter than in the classical cannabinoids where a seven-carbon heptyl chain produces the most potent compounds. This difference is thought to reflect a slightly different binding conformation adopted by the naphthoylindole compounds as compared to the classical cannabinoids, and may be useful in characterizing the active site of the CB1 and CB2 receptors.

MDA-19 (also known as BZO-HEXOXIZID) is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with reasonable selectivity over the psychoactive CB1 receptor, though with some variation between species. In animal studies it was effective for the treatment of neuropathic pain, but did not affect rat locomotor activity in that specific study. The pharmacology of MDA-19 in rat cannabinoid receptors have been demonstrated to function differently than human cannabinoid receptors with MDA-19 binding to human CB1 receptors 6.9× higher than rat CB1 receptors.
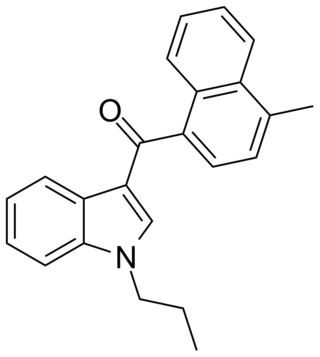
JWH-120 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-propyl analog of JWH-122. It is a potent and selective ligand for the CB2 receptor, but a weaker ligand for the CB1 receptor. It has a binding affinity of Ki = 6.1 ± 0.7 nM at the CB2 subtype and 173 times selectivity over the CB1 subtype.

JWH-369 ((5-(2-chlorophenyl)-1-pentyl-1H-pyrrol-3-yl)(naphthalen-1-yl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent agonist of the CB1 (Ki = 7.9 ± 0.4nM) and CB2 (Ki = 5.2 ± 0.3nM) receptors, with a slight selectivity for the latter. JWH-369 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-373 ([5-(2-butylphenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 60 ± 3nM) and CB2 (Ki = 69 ± 2nM) receptors, with a slight selectivity for the former. JWH-373 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-309 (naphthalen-1-yl-(5-naphthalen-1-yl-1-pentylpyrrol-3-yl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 41 ± 3nM) and CB2 (Ki = 49 ± 7nM) receptors, displaying a slight selectivity for the former. JWH-309 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.
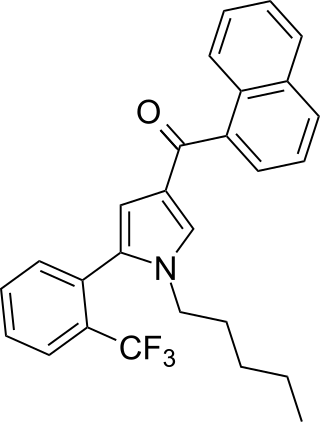
JWH-372 (naphthalen-1-yl-[1-pentyl-5-[2-(trifluoromethyl)phenyl]pyrrol-3-yl]methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent and selective agonist of the CB2 receptor. JWH-372 binds approximately 9 times stronger to the CB2 receptor (Ki = 8.2 ± 0.2nM) than the CB1 receptor (Ki = 77 ± 2nM). The selectivity of JWH-372 for the CB2 receptor is likely due to the electron-withdrawing character of the trifluoromethyl group rather than steric effects, as the o-methyl compound JWH-370 was only mildly selective for the CB2 receptor (CB1 Ki = 5.6 ± 0.4nM, CB2 Ki = 4.0 ± 0.5nM).

JWH-370 ([5-(2-methylphenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 5.6 ± 0.4nM) and CB2 (Ki = 4.0 ± 0.5nM) receptors, with a slight selectivity for the CB2 receptor. JWH-370 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-368 ([5-(3-Fluorophenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 16 ± 1nM) and CB2 (Ki = 9.1 ± 0.7nM) receptors, binding ~1.76 times stronger to the CB2 receptor than to the CB1 receptor. JWH-368 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-367 ([5-(3-methoxyphenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 53 ± 2nM) and CB2 (Ki = 23 ± 1nM) receptors, binding ~2.3 times stronger to the CB2 receptor than to the CB1 receptor. JWH-367 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-366 (naphthalen-1-yl-(1-pentyl-5-pyridin-3-ylpyrrol-3-yl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 191 ± 12nM) and CB2 (Ki = 24 ± 1nM) receptors, with a strong (~8x) selectivity for the CB2 receptor over the CB1 receptor. JWH-366 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-365 ((5-(2-Ethylphenyl)-1-pentyl-1H-pyrrol-3-yl)(naphthalen-1-yl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 17 ± 1nM) and CB2 (Ki = 3.4 ± 0.2nM) receptors, with a strong (~5x) selectivity for the CB2 receptor over the CB1 receptor. JWH-365 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-364 ([5-(4-Ethylphenyl)-1-pentyl-1H-pyrrol-3-yl](1-naphthyl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 34 ± 3nM) and CB2 (Ki = 29 ± 1nM) receptors, with a slight selectivity for the latter. JWH-364 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-363 (1-Naphthyl{1-pentyl-5-[3-(trifluoromethyl)phenyl]-1H-pyrrol-3-yl}methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 245 ± 5nM) and CB2 (Ki = 71 ± 1nM) receptors, with a moderate (~3.45x) selectivity for the latter. JWH-363 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.
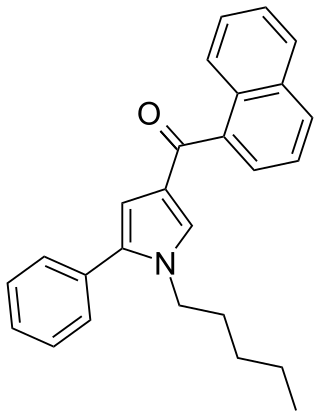
JWH-145 (1-naphthalenyl(1-pentyl-5-phenyl-1H-pyrrol-3-yl)-methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 14 ± 2nM) and CB2 (Ki = 6.4 ± 0.4nM) receptors, with a moderate (~2.2x) selectivity for the CB2 receptor. JWH-145 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-146 (1-heptyl-5-phenyl-1H-pyrrol-3-yl)-1-naphthalenyl-methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 21 ± 2nM) and CB2 (Ki = 62 ± 5nM) receptors, with a moderate (~2.9x) selectivity for the CB1 receptor over the CB2 receptor. JWH-146 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-150 ((1-butyl-5-phenylpyrrol-3-yl)-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 60 ± 1nM) and CB2 (Ki = 15 ± 2nM) receptors, with a moderate four-fold selectivity for the CB2 receptor. JWH-150 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.




















