Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.
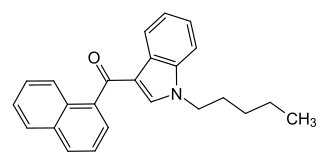
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends".

BAY 59-3074 is a drug which is a cannabinoid receptor partial agonist developed by Bayer AG. It has analgesic effects and is used in scientific research. It is orally active in animals, and has modest affinity for both CB1 and CB2 receptors, with Ki values of 48.3nM at CB1 and 45.5nM at CB2.
Originally synthesized by chemist Wayne E. Kenney, BAY 38-7271 (KN 38-7271) is a drug which is a cannabinoid receptor agonist developed by Bayer AG. It has analgesic and neuroprotective effects and is used in scientific research, with proposed uses in the treatment of traumatic brain injury. It is a full agonist with around the same potency as CP 55,940 in animal studies, and has fairly high affinity for both CB1 and CB2 receptors, with Ki values of 2.91nM at CB1 and 4.24nM at CB2. It has been licensed to KeyNeurotek Pharmaceuticals for clinical development, and was in Phase II trials in 2008 but its development appears to have stopped.

O-1057 is an analgesic cannabinoid derivative created by Organix Inc., Newburyport, Massachusetts, for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many related cannabinoids. It has moderate affinity for both CB1 and CB2 receptors, with Ki values of 8.36 nM at CB1 and 7.95 nM at CB2

VCHSR is a drug used in scientific research which acts as a selective antagonist of the cannabinoid receptor CB1. It is derived from the widely used CB1 antagonist rimonabant, and has similar potency and selectivity for the CB1 receptor, but has been modified to remove the hydrogen bonding capability in the C-3 substituent region, which removes the inverse agonist effect that rimonabant produces at high doses, so that VCHSR instead acts as a neutral antagonist, blocking the receptor but producing no physiological effect of its own.

AMG-1 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC with a rigidified and extended 3-position side chain. AMG-1 is a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 0.6 nM at CB1 vs 3.1 nM at CB2.
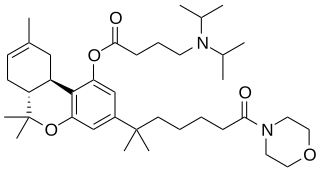
O-2694 is a drug that is a cannabinoid derivative. It has analgesic effects and is used in scientific research. Unlike most cannabinoids discovered to date, it is highly water-soluble, which gives it considerable advantages over many related drugs. It has high affinity for both CB1 and CB2 receptors, with Ki values of 3.7 nM at CB1 and 2.8 nM at CB2. However, it has complex pharmacokinetics as most of the administered dose is metabolised by hydrolysis of the ester link to the water-insoluble compound O-2372, thus producing a biphasic effects profile that is less suitable for research purposes than the related compound O-2545.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

A-796,260 is a drug developed by Abbott Laboratories that acts as a potent and selective cannabinoid CB2 receptor agonist. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group, imparts significant selectivity for CB2, and A-796,260 was found to be a highly selective CB2 agonist with little affinity for CB1, having a CB2Ki of 4.6 nM vs 945 nM at CB1. It has potent analgesic and anti-inflammatory actions in animal models, being especially effective in models of neuropathic pain, but without producing cannabis-like behavioral effects.

SER-601 (COR-167) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, based on a quinolone-3-carboxylic acid core structure, with 190 times selectivity for CB2 over the related CB1 receptor. It has analgesic effects in animal studies, as well as neuroprotective effects, but without a "cannabis high" due to its low affinity for CB1. A number of related compounds are known, almost all of which have high selectivity for CB2.
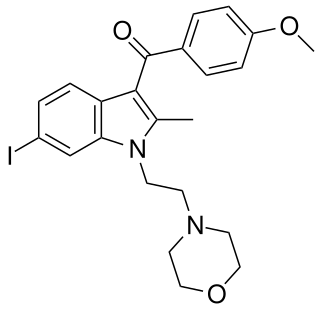
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

Org 28312 is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, but did not proceed to human trials, with the related compound Org 28611 chosen instead due to its better penetration into the brain. The structure-activity relationships of these compounds have subsequently been investigated further leading to the development of a number of more potent analogues, derived by cyclisation around the indole or piperazine rings.
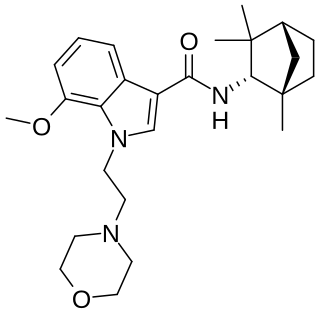
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.

CBS-0550 is a drug developed by Taisho Pharmaceutical, which acts as a potent and selective cannabinoid CB2 receptor agonist, with 1400x selectivity for CB2 over the related CB1 receptor. Unlike most cannabinoid agonists, CBS-0550 has good solubility in water, and in animal studies it was found to produce analgesic and anti-hyperalgesic effects. A number of related compounds have been developed with similar properties.
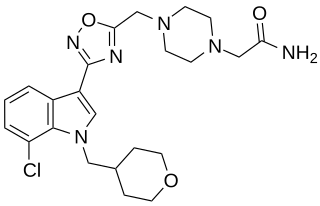
LBP-1 is a drug originally developed by Organon for the treatment of neuropathic pain, It acts as a potent and selective cannabinoid receptor agonist, with high potency at both the CB1 and CB2 receptors, but low penetration of the blood–brain barrier. This makes LBP-1 peripherally selective, and while it was effective in animal models of neuropathic pain and allodynia, it did not produce cannabinoid-appropriate responding suggestive of central effects, at any dose tested.

AZD-1940 is a drug developed by AstraZeneca, that is a peripherally selective cannabinoid agonist which binds with high affinity to both the CB1 and CB2 receptors. It was developed for the treatment of neuropathic pain, but while it showed good peripheral selectivity in animal studies, in human clinical trials it failed to show sufficient analgesic efficacy and produced unexpectedly strong side effects associated with central cannabinoid activity, and so was discontinued from further development.

CUMYL-FUBINACA (SGT-149) is an indazole-3-carboxamide based synthetic cannabinoid receptor agonist, with an EC50 of 1.8nM for human CB1 receptors and 23.7nM for human CB2 receptors, giving it around 13x selectivity for CB1. It has been sold online as a designer drug.