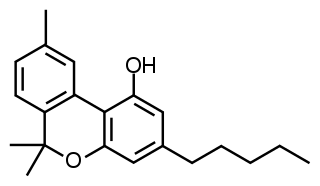
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.

Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activate the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

O-1057 is an analgesic cannabinoid derivative created by Organix Inc., Newburyport, Massachusetts, for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many related cannabinoids. It has moderate affinity for both CB1 and CB2 receptors, with Ki values of 8.36 nM at CB1 and 7.95 nM at CB2

JWH-030 is a research chemical which is a cannabinoid receptor agonist. It has analgesic effects and is used in scientific research. It is a partial agonist at CB1 receptors, with a Ki of 87 nM, making it roughly half the potency of THC. It was discovered and named after John W. Huffman.
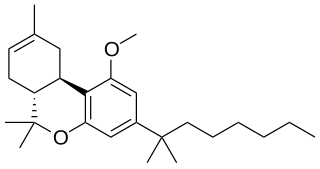
L-759,633 is an analgesic drug that is a cannabinoid agonist. It is a fairly selective agonist for the CB2 receptor, with selectivity of 163x for CB2 over CB1.

L-759,656 is an analgesic drug that is a cannabinoid agonist. It is a highly selective agonist for the CB2 receptor, with selectivity of 414x for CB2 over CB1, although it is still not as selective as newer agents such as HU-308.

LY-320,135 is a drug used in scientific research which acts as a selective antagonist of the cannabinoid receptor CB1. It was developed by Eli Lilly and Company in the 1990s.

AMG-1 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC with a rigidified and extended 3-position side chain. AMG-1 is a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 0.6 nM at CB1 vs 3.1 nM at CB2.

O-823 is a drug which is a cannabinoid derivative that is used in scientific research. It is described as a mixed agonist/antagonist at the cannabinoid receptor CB1, meaning that it acts as an antagonist when co-administered alongside a more potent CB1 agonist, but exhibits weak partial agonist effects when administered by itself.
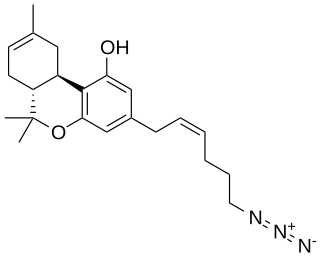
O-1238 is a drug which is a cannabinoid derivative that is used in scientific research. It is a partial agonist at the cannabinoid receptor CB1, producing a maximal stimulation of 58.3% with a Ki of 8.45 nM.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

Org 27569 is a drug which acts as a potent and selective negative allosteric modulator of the cannabinoid CB1 receptor. Studies in vitro suggest that it binds to a regulatory site on the CB1 receptor target, causing a conformational change that increases the binding affinity of CB1 agonists such as CP 55,940, while decreasing the binding affinity of CB1 antagonists or inverse agonists such as rimonabant. However while Org 27569 increases the ability of CB1 agonists to bind to the receptor, it decreases their efficacy at stimulating second messenger signalling once bound, and so in practice behaves as an insurmountable antagonist of CB1 receptor function.
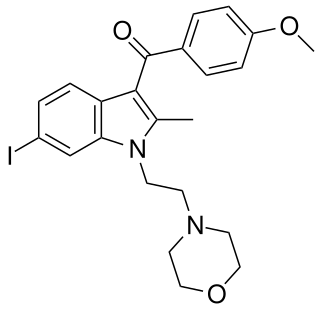
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.
O-2050 is a drug that is a classical cannabinoid derivative, which acts as an antagonist for the CB1 receptor. This gives it an advantage in research over many commonly used cannabinoid antagonists, such as rimonabant, which at higher doses act as inverse agonists at CB1 as well as showing off-target effects. However, while O-2050 acts as a silent antagonist in vitro, some tests in vivo have suggested it may show agonist activity under certain circumstances.

AM-6545 is a drug which acts as a peripherally selective silent antagonist for the CB1 receptor, and was developed for the treatment of obesity. Other cannabinoid antagonists such as rimonabant have been marketed for this application, but have subsequently been withdrawn from sale because of centrally mediated side effects such as depression and nausea. Because AM-6545 does not cross the blood–brain barrier to any significant extent, it does not produce these kinds of side effects, but has still been shown to effectively reduce appetite and food consumption in animal studies.


















