The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. [1] [2] [3] [4] Some of those are:
Contents
| Name | Class | Ki / nM at CB1 | Ki / nM at CB2 | Selectivity | Structure |
|---|---|---|---|---|---|
| JWH-004 | Naphthoylindole | 48 ± 13 | 4 ± 1.5 | CB2 (12x) |  |
| JWH-007 [5] | Naphthoylindole | 9.5 ± 4.5 | 2.9 ± 2.6 | CB2 (3.3x) | 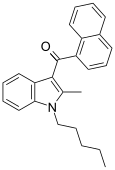 |
| JWH-009 | Naphthoylindole | >10000 | 141 ± 14 | CB2 (>70x) | 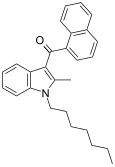 |
| JWH-011 | Naphthoylindole | 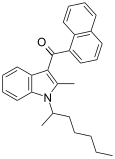 | |||
| JWH-015 [5] | Naphthoylindole | 164 ± 22 | 13.8 ± 4.6 | CB2 (12x) |  |
| JWH-016 | Naphthoylindole | 22 ± 1.5 | 4.3 ± 1.6 | CB2 (5.1x) | 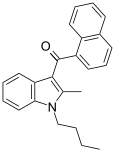 |
| JWH-018 [5] | Naphthoylindole | 9 ± 5 | 2.9 ± 2.6 | CB2 (3.1x) |  |
| JWH-019 | Naphthoylindole | 9.8 ± 2 | 5.55 ± 2 | CB2 (1.77x) |  |
| JWH-020 | Naphthoylindole | 128 ± 17 | 205 ± 20 | CB1 (1.6x) | 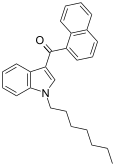 |
| JWH-030 | Naphthoylpyrrole | 87 ± 3 | 320 ± 127 | CB1 (3.7x) |  |
| JWH-031 | Naphthoylpyrrole | 399 ± 109 | 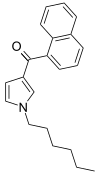 | ||
| JWH-032 | Naphthoylpyrrole | >10000 | >10000 | — |  |
| JWH-033 | Naphthoylpyrrole | 666 ± 77 | 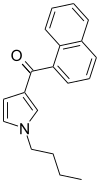 | ||
| JWH-036 | Naphthoylpyrrole | 309 ± 11 | 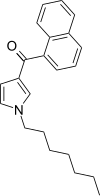 | ||
| JWH-042 [6] | Naphthoylindole | >10000 | 5050 ± 192 | CB2 | 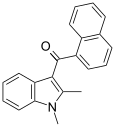 |
| JWH-043 [6] | Naphthoylindole | 1180 ± 44 | 964 ± 242 | CB2 (1.2x) |  |
| JWH-044 | Naphthoylpyrrole | >10000 | >10000 | — | 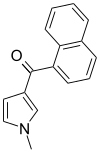 |
| JWH-045 | Naphthoylpyrrole | >10000 | >10000 | — |  |
| JWH-046 [6] | Naphthoylindole | 343 ± 38 | 16.3 ± 4.9 | CB2 (21x) |  |
| JWH-047 [6] | Naphthoylindole | 59 ± 3 | 3.47 ± 1.80 | CB2 (17x) | 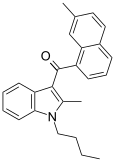 |
| JWH-048 [6] | Naphthoylindole | 10.7 ± 1.0 | 0.49 ± 0.13 | CB2 (22x) |  |
| JWH-049 [6] | Naphthoylindole | 55.1 ± 17.0 | 32.3 ± 2.4 | CB2 (1.7x) |  |
| JWH-050 [6] | Naphthoylindole | 342 ± 6 | 526 ± 133 | CB1 (1.5x) |  |
| JWH-051 | Dibenzopyran | 1.20 | 0.03 | CB2 (40x) |  |
| JWH-056 [7] | Dibenzopyran | >10000 | 32 ± 9 | CB2 |  |
| JWH-057 [8] | Dibenzopyran | 23 ± 7 | 2.9 ± 1.6 | CB2 (8x) |  |
| JWH-065 [7] | Dibenzopyran | 399 ± 76 | 10 ± 2 | CB2 (40x) |  |
| JWH-070 [6] | Naphthoylindole | >10000 | >10000 | 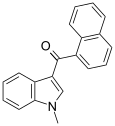 | |
| JWH-071 [6] | Naphthoylindole | 1340 ± 123 | 2940 ± 852 | CB1 (2.2x) |  |
| JWH-072 | Naphthoylindole | 1050 ± 5.5 | 170 ± 54 | CB2 (6x) |  |
| JWH-073 | Naphthoylindole | 8.9 ± 1.8 | 27 ± 12 | CB1 (3x) |  |
| JWH-076 [5] | Naphthoylindole | 214 ± 11 | 106 ± 46 | CB2 (2x) |  |
| JWH-077 [6] | Naphthoylindole | >10000 | >10000 |  | |
| JWH-078 [6] | Naphthoylindole | 817 ± 60 | 633 ± 116 | CB2 (1.3x) |  |
| JWH-079 [6] | Naphthoylindole | 63.0 ± 3.0 | 32.0 ± 6.0 | CB2 (2x) |  |
| JWH-080 [6] | Naphthoylindole | 8.9 ± 1.8 | 2.21 ± 1.30 | CB2 (4x) |  |
| JWH-081 [6] | Naphthoylindole | 1.2 ± 0.03 | 12.4 ± 2.2 | CB1 (10x) |  |
| JWH-082 [6] | Naphthoylindole | 5.3 ± 0.8 | 6.40 ± 0.94 | CB1 (1.2x) |  |
| JWH-083 [6] | Naphthoylindole | 106 ± 12 | 102 ± 50 | — |  |
| JWH-091 [9] (Δ8-THCP) | Dibenzopyran | 22.0 ± 3.9 |  | ||
| JWH-093 [6] | Naphthoylindole | 40.7 ± 2.8 | 59.1 ± 10.5 | CB1 (1.45x) |  |
| JWH-094 [6] | Naphthoylindole | 476 ± 67 | 97.3 ± 2.7 | CB2 (4.9x) |  |
| JWH-095 [6] | Naphthoylindole | 140 ± 4.3 | 312 ± 83 | CB1 (2.2x) |  |
| JWH-096 [6] | Naphthoylindole | 33.7 ± 2.9 | 13.3 ± 5.6 | CB2 (2.5x) |  |
| JWH-097 [6] | Naphthoylindole | 455 ± 28 | 121 ± 15 | CB2 (3.8x) |  |
| JWH-098 [6] | Naphthoylindole | 4.5 ± 0.1 | 1.9 ± 0.3 | CB2 (2.4x) |  |
| JWH-099 [6] | Naphthoylindole | 35.3 ± 9.0 | 17.8 ± 2.9 | CB2 (2x) |  |
| JWH-100 [6] | Naphthoylindole | 381 ± 102 | 155 ± 74 | CB2 (2.5x) |  |
| JWH-102 [7] | Dibenzopyran | 7.9 ± 0.9 | 5.2 ± 2.0 | CB2 (1.5x) |  |
| JWH-103 [7] | Dibenzopyran | 28 ± 3 | 23 ± 7 | CB2 (1.2x) |  |
| JWH-116 [10] | Naphthoylindole | 52 ± 5 |  | ||
| JWH-120 [5] | Naphthoylindole | 1054 ± 31 | 6.1 ± 0.7 | CB2 (173x) |  |
| JWH-122 [10] | Naphthoylindole | 0.69 ± 0.05 | 1.2 ± 1.2 | — |  |
| JWH-124 (Δ8-Parahexyl) | Dibenzopyran | 41.0 ± 3.8 | 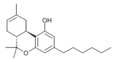 | ||
| JWH-130 (Δ8-THCB) | Dibenzopyran | 65.0 ± 13 |  | ||
| JWH-133 [7] | Dibenzopyran | 677 ± 132 | 3.4 ± 1.0 | CB2 (200x) |  |
| JWH-138 [11] | Dibenzopyran | 8.5 ± 1.4 |  | ||
| JWH-139 [12] | Dibenzopyran | 2290 ± 505 | 14 ± 10 | CB2 (164x) |  |
| JWH-142 [7] | Dibenzopyran | 529 ± 49 | 35 ± 14 | CB2 (15x) | 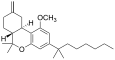 |
| JWH-143 [7] | Dibenzopyran | 924 ± 104 | 65 ± 8 | CB2 (14x) |  |
| JWH-145 [13] | Naphthoylpyrrole | 14 ± 2 | 6.4 ± 0.4 | CB2 (2.2x) |  |
| JWH-146 [13] | Naphthoylpyrrole | 21 ± 2 | 62 ± 5 | CB2 (3.0x) |  |
| JWH-147 [13] | Naphthoylpyrrole | 11 ± 1 | 7.1 ± 0.2 | CB2 (1.5x) |  |
| JWH-148 [5] | Naphthoylindole | 123 ± 8 | 14.0 ± 1.0 | CB2 (8x) |  |
| JWH-149 [5] | Naphthoylindole | 5.0 ± 2.1 | 0.73 ± 0.03 | CB2 (6.8x) |  |
| JWH-150 [13] | Naphthoylpyrrole | 60 ± 1 | 15 ± 2 | CB2 (4x) | 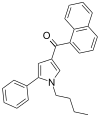 |
| JWH-151 [5] | Naphthoylindole | >10000 | 30 ± 1.1 | CB2 (>333x) |  |
| JWH-153 [5] | Naphthoylindole | 250 ± 24 | 11 ± 0.5 | CB2 (23x) |  |
| JWH-156 [13] | Naphthoylpyrrole | 404 ± 18 | 104 ± 18 | CB2 (4x) |  |
| JWH-159 [5] | Naphthoylindole | 45 ± 1 | 10.4 ± 1.4 | CB2 (4.3x) |  |
| JWH-160 [5] | Naphthoylindole | 1568 ± 201 | 441 ± 110 | CB2 (3.6x) |  |
| JWH-161 | Dibenzopyran hybrid | 19.0 | 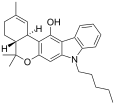 | ||
| JWH-163 [5] | Naphthoylindole | 2358 ± 215 | 138 ± 12 | CB2 (17x) |  |
| JWH-164 [5] | Naphthoylindole | 6.6 ± 0.7 | 6.9 ± 0.2 | — |  |
| JWH-165 [5] | Naphthoylindole | 204 ± 26 | 71 ± 8 | CB2 (2.9x) | 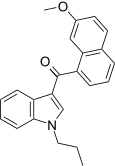 |
| JWH-166 [5] | Naphthoylindole | 44 ± 10 | 1.9 ± 0.08 | CB2 (23x) | 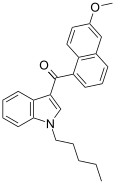 |
| JWH-167 | Phenylacetylindole | 90 ± 17 | 159 ± 14 | CB1 (1.77x) | 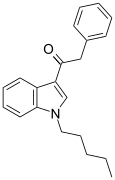 |
| JWH-171 | Hydrocarbon | 51 |  | ||
| JWH-175 [10] | Naphthylmethylindole | 22 ± 2 |  | ||
| JWH-176 [10] | Hydrocarbon | 26 ± 4 |  | ||
| JWH-180 [5] | Naphthoylindole | 26 ± 2 | 9.6 ± 2.0 | CB2 (2.7x) |  |
| JWH-181 [5] | Naphthoylindole | 1.3 ± 0.1 | 0.62 ± 0.04 | CB2 (2.1x) |  |
| JWH-182 [5] | Naphthoylindole | 0.65 ± 0.03 | 1.1 ± 0.1 | CB1 (1.7x) |  |
| JWH-184 [10] | Naphthylmethylindole | 23 ± 6 |  | ||
| JWH-185 [10] | Naphthylmethylindole | 17 ± 3 |  | ||
| JWH-186 [14] | Dibenzopyran | 187 ± 23 | 5.6 ± 1.7 | CB2 (33x) | 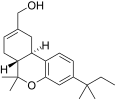 |
| JWH-187 [14] | Dibenzopyran | 84 ± 16 | 3.4 ± 0.5 | CB2 (25x) | 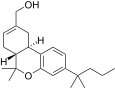 |
| JWH-188 [14] | Dibenzopyran | 270 ± 58 | 18 ± 2 | CB2 (15x) | 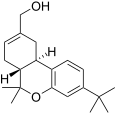 |
| JWH-189 [5] | Naphthoylindole | 52 ± 2 | 12 ± 0.8 | CB2 (4.3x) | 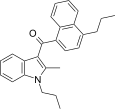 |
| JWH-190 [14] | Dibenzopyran | 8.8 ± 1.4 | 1.6 ± 0.03 | CB2 (5.5x) | 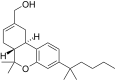 |
| JWH-191 [14] | Dibenzopyran | 1.8 ± 0.3 | 0.52 ± 0.03 | CB2 (3.5x) |  |
| JWH-192 [10] | Naphthylmethylindole | 41 ± 13 | 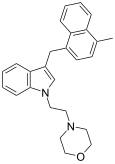 | ||
| JWH-193 [10] | Naphthoylindole | 6 ± 1 |  | ||
| JWH-194 [10] | Naphthylmethylindole | 127 ± 19 |  | ||
| JWH-195 [10] | Naphthylmethylindole | 113 ± 28 |  | ||
| JWH-196 [10] | Naphthylmethylindole | 151 ± 18 |  | ||
| JWH-197 [10] | Naphthylmethylindole | 323 ± 98 |  | ||
| JWH-198 [10] | Naphthoylindole | 10 ± 2 |  | ||
| JWH-199 [10] | Naphthylmethylindole | 20 ± 2 |  | ||
| JWH-200 [10] | Naphthoylindole | 42 ± 5 | 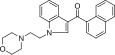 | ||
| JWH-201 [15] | Phenylacetylindole | 1064 ± 21 | 444 ± 14 | CB2 (2.4x) |  |
| JWH-202 [15] | Phenylacetylindole | 1678 ± 63 | 645 ± 6 | CB2 (2.6x) |  |
| JWH-203 [15] | Phenylacetylindole | 8.0 ± 0.9 | 7.0 ± 1.3 | — | 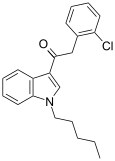 |
| JWH-204 [15] | Phenylacetylindole | 13 ± 1 | 25 ± 1 | CB1 (1.9x) |  |
| JWH-205 [15] | Phenylacetylindole | 124 ± 23 | 180 ± 9 | CB1 (1.45x) | 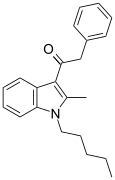 |
| JWH-206 [15] | Phenylacetylindole | 389 ± 25 | 498 ± 37 | CB1 (1.28x) |  |
| JWH-207 [15] | Phenylacetylindole | 1598 ± 134 | 3723 ± 10 | CB1 (2.33x) |  |
| JWH-208 [15] | Phenylacetylindole | 179 ± 7 | 570 ± 127 | CB1 (3.18x) |  |
| JWH-209 [15] | Phenylacetylindole | 746 ± 49 | 1353 ± 270 | CB1 (1.81x) |  |
| JWH-210 [5] | Naphthoylindole | 0.46 ± 0.03 | 0.69 ± 0.01 | CB1 (1.5x) |  |
| JWH-211 [5] | Naphthoylindole | 70 ± 0.8 | 12 ± 0.8 | CB2 (5.8x) |  |
| JWH-212 [5] | Naphthoylindole | 33 ± 0.9 | 10 ± 1.2 | CB2 (3.3x) |  |
| JWH-213 [5] | Naphthoylindole | 1.5 ± 0.2 | 0.42 ± 0.05 | CB2 (3.6x) |  |
| JWH-215 [14] | Dibenzopyran | 1008 ± 117 | 85 ± 21 | CB2 (12x) |  |
| JWH-216 [14] | Dibenzopyran | 1856 ± 148 | 333 ± 104 | CB2 (5.6x) |  |
| JWH-217 [14] | Dibenzopyran | >10000 | 1404 ± 66 | CB2 (>7x) |  |
| JWH-220 | Hydrocarbon | 19 |  | ||
| JWH-224 [14] | Dibenzopyran | 347 ± 34 | 28 ± 1 | CB2 (12.3x) | 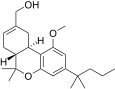 |
| JWH-225 [14] | Dibenzopyran | >10000 | 325 ± 70 | CB2 (>31x) | 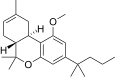 |
| JWH-226 [14] | Dibenzopyran | 4001 ± 282 | 43 ± 3 | CB2 (93x) |  |
| JWH-227 [14] | Dibenzopyran | 40 ± 6 | 4.4 ± 0.3 | CB2 (9x) |  |
| JWH-229 [16] | Dibenzopyran | 3134 ± 110 | 18 ± 2 | CB2 (174x) |  |
| JWH-230 [14] | Dibenzopyran | 15 ± 3 | 1.4 ± 0.12 | CB2 (10.7x) |  |
| JWH-233 [14] | Dibenzopyran | 14 ± 3 | 1.0 ± 0.3 | CB2 (14x) |  |
| JWH-234 [5] | Naphthoylindole | 8.4 ± 1.8 | 3.8 ± 0.6 | CB2 (2.2x) | 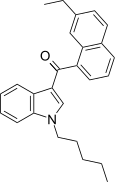 |
| JWH-235 [5] | Naphthoylindole | 338 ± 34 | 123 ± 34 | CB2 (2.7x) | 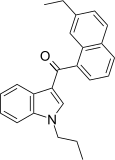 |
| JWH-236 [5] | Naphthoylindole | 1351 ± 204 | 240 ± 63 | CB2 (5.6x) | 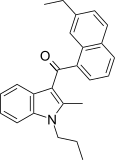 |
| JWH-237 [15] | Phenylacetylindole | 38 ± 10 | 106 ± 2 | CB1 (2.8x) |  |
| JWH-239 [5] | Naphthoylindole | 342 ± 20 | 52 ± 6 | CB2 (6.6x) | 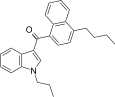 |
| JWH-240 [5] | Naphthoylindole | 14 ± 1 | 7.2 ± 1.3 | CB2 (1.9x) | 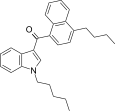 |
| JWH-241 [5] | Naphthoylindole | 147 ± 20 | 49 ± 7 | CB2 (3.0x) | 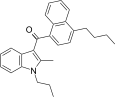 |
| JWH-242 [5] | Naphthoylindole | 42 ± 9 | 6.5 ± 0.3 | CB2 (6.5x) | 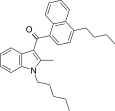 |
| JWH-243 [13] | Naphthoylpyrrole | 285 ± 40 | 41 ± 3 | CB2 (6.95x) |  |
| JWH-244 [13] | Naphthoylpyrrole | 130 ± 6 | 18 ± 1 | CB2 (7.22x) |  |
| JWH-245 [13] | Naphthoylpyrrole | 276 ± 4 | 25 ± 2 | CB2 (11x) |  |
| JWH-246 [13] | Naphthoylpyrrole | 70 ± 4 | 16 ± 1 | CB2 (4.38x) |  |
| JWH-247 [14] | Dibenzopyran | 427 ± 31 | 99 ± 4 | CB2 (4.3x) | 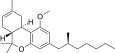 |
| JWH-248 [15] | Phenylacetylindole | 1028 ± 39 | 657 ± 19 | CB2 (1.56x) |  |
| JWH-249 [15] | Phenylacetylindole | 8.4 ± 1.8 | 20 ± 2 | CB1 (2.38x) | 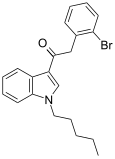 |
| JWH-250 [15] | Phenylacetylindole | 11 ± 2 | 33 ± 2 | CB1 (3x) |  |
| JWH-251 [15] | Phenylacetylindole | 29 ± 3 | 146 ± 36 | CB2 (5x) |  |
| JWH-252 [15] | Phenylacetylindole | 23 ± 3 | 19 ± 1 | CB2 (1.2x) |  |
| JWH-253 [15] | Phenylacetylindole | 62 ± 10 | 84 ± 12 | CB1 (1.35x) |  |
| JWH-254 [14] | Dibenzopyran | 4724 ± 509 | 319 ± 16 | CB2 (14.8x) |  |
| JWH-256 [14] | Dibenzopyran | 4300 ± 888 | 97 ± 18 | CB2 (44x) | 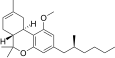 |
| JWH-258 [5] | Naphthoylindole | 4.6 ± 0.6 | 10.5 ± 1.3 | CB1 (2.3x) | 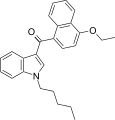 |
| JWH-259 [5] | Naphthoylindole | 220 ± 29 | 74 ± 7 | CB2 (3.0x) |  |
| JWH-260 [5] | Naphthoylindole | 29 ± 0.4 | 25 ± 1.9 | CB2 (1.2x) |  |
| JWH-261 [5] | Naphthoylindole | 767 ± 105 | 221 ± 14 | CB2 (3.5x) | 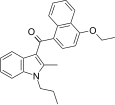 |
| JWH-262 [5] | Naphthoylindole | 28 ± 3 | 5.6 ± 0.7 | CB2 (5.0x) |  |
| JWH-265 [5] | Naphthoylindole | 3788 ± 323 | 80 ± 13 | CB2 (47x) | 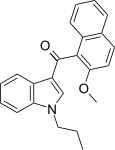 |
| JWH-266 [5] | Naphthoylindole | >10000 | 455 ± 55 | CB2 (>22x) |  |
| JWH-267 [5] | Naphthoylindole | 381 ± 16 | 7.2 ± 0.14 | CB2 (53x) | 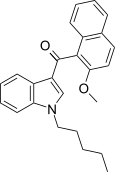 |
| JWH-268 [5] | Naphthoylindole | 1379 ± 193 | 40 ± 0.6 | CB2 (34x) |  |
| JWH-277 [14] | Dibenzopyran | 3905 ± 91 | 589 ± 65 | CB2 (6.6x) |  |
| JWH-278 [14] | Dibenzopyran | 906 ± 80 | 69 ± 6 | CB2 (13x) |  |
| JWH-292 [13] | Naphthoylpyrrole | 29 ± 1 | 20 ± 1 | CB2 (1.45x) |  |
| JWH-293 [13] | Naphthoylpyrrole | 100 ± 5 | 41 ± 4 | CB2 (2.44x) |  |
| JWH-298 [14] | Dibenzopyran | 812 ± 67 | 198 ± 23 | CB2 (4.1x) |  |
| JWH-299 [14] | Dibenzopyran | 415 ± 50 | 30 ± 2 | CB2 (13.8x) |  |
| JWH-300 [12] | Dibenzopyran | 118 ± 16 | 5.3 ± 0.1 | CB2 (22x) |  |
| JWH-301 [14] | Dibenzopyran | 295 ± 64 | 48 ± 4 | CB2 (6.1x) |  |
| JWH-302 [15] | Phenylacetylindole | 17 ± 2 | 89 ± 15 | CB1 (5.26x) |  |
| JWH-303 [15] | Phenylacetylindole | 117 ± 10 | 138 ± 12 | CB1 (1.18x) | 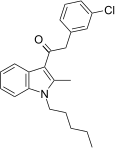 |
| JWH-304 [15] | Phenylacetylindole | 3363 ± 332 | 2679 ± 688 | CB2 (1.26x) |  |
| JWH-305 [15] | Phenylacetylindole | 15 ± 1.8 | 29 ± 5 | CB1 (1.93x) |  |
| JWH-306 [15] | Phenylacetylindole | 25 ± 1 | 82 ± 11 | CB1 (3.28x) |  |
| JWH-307 [13] | Naphthoylpyrrole | 7.7 ± 1.8 | 3.3 ± 0.2 | CB2 (2.33x) |  |
| JWH-308 [13] | Naphthoylpyrrole | 41 ± 1 | 33 ± 2 | CB2 (1.24x) |  |
| JWH-309 [13] | Naphthoylpyrrole | 41 ± 3 | 49 ± 7 | CB1 (1.20x) |  |
| JWH-310 [14] | Dibenzopyran | 1059 ± 51 | 36 ± 3 | CB2 (29x) |  |
| JWH-311 [15] | Phenylacetylindole | 23 ± 2 | 39 ± 3 | CB1 (1.70x) | 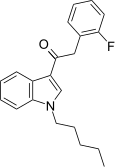 |
| JWH-312 [15] | Phenylacetylindole | 72 ± 7 | 91 ± 20 | CB1 (1.26x) |  |
| JWH-313 [15] | Phenylacetylindole | 422 ± 19 | 365 ± 92 | CB2 (1.16x) |  |
| JWH-314 [15] | Phenylacetylindole | 39 ± 2 | 76 ± 4 | CB1 (1.95x) |  |
| JWH-315 [15] | Phenylacetylindole | 430 ± 24 | 182 ± 23 | CB2 (3.36x) |  |
| JWH-316 [15] | Phenylacetylindole | 2862 ± 670 | 781 ± 105 | CB2 (3.66x) | 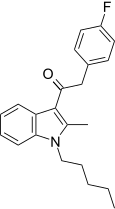 |
| JWH-336 [12] | Dibenzopyran | 4589 ± 367 | 153 ± 15 | CB2 (30x) |  |
| JWH-338 [14] | Dibenzopyran | >10000 | 111 ± 16 | CB2 (>90x) | 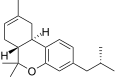 |
| JWH-339 [14] | Dibenzopyran | >10000 | 2317 ± 93 | CB2 (>4.3x) | 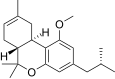 |
| JWH-340 [14] | Dibenzopyran | 135 ± 6 | 30 ± 1 | CB2 (4.5x) |  |
| JWH-341 [14] | Dibenzopyran | 100 ± 8 | 10 ± 0.1 | CB2 (10x) | 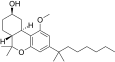 |
| JWH-346 [13] | Naphthoylpyrrole | 67 ± 6 | 39 ± 2 | CB2 (1.72x) |  |
| JWH-347 [13] | Naphthoylpyrrole | 333 ± 17 | 169 ± 17 | CB2 (1.97x) |  |
| JWH-348 [13] | Naphthoylpyrrole | 218 ± 19 | 53 ± 1 | CB2 (4.11x) | 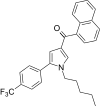 |
| JWH-349 [14] | Dibenzopyran | 376 ± 1 | 38 ± 4 | CB2 (9.9x) |  |
| JWH-350 [12] | Dibenzopyran | 395 ± 50 | 12 ± 1 | CB2 (33x) | 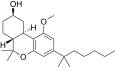 |
| JWH-351 [14] | Dibenzopyran | >10000 | 295 ± 3 | CB2 (>34x) | 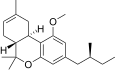 |
| JWH-352 [14] | Dibenzopyran | >10000 | 47 ± 2 | CB2 (>213x) | 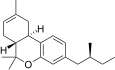 |
| JWH-353 [14] | Dibenzopyran | 1493 ± 10 | 31 ± 1 | CB2 (48x) |  |
| JWH-354 [14] | Dibenzopyran | 1961 ± 21 | 241 ± 14 | CB2 (8.1x) |  |
| JWH-355 [14] | Dibenzopyran | 2162 ± 220 | 108 ± 17 | CB2 (20x) |  |
| JWH-356 [14] | Dibenzopyran | 5837 ± 701 | 108 ± 17 | CB2 (54x) |  |
| JWH-357 [14] | Dibenzopyran | 647 ± 78 | 185 ± 4 | CB2 (3.5x) | 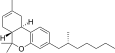 |
| JWH-358 [14] | Dibenzopyran | 1243 ± 266 | 52 ± 3 | CB2 (24x) | 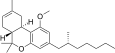 |
| JWH-359 | Dibenzopyran | 2918 ± 450 | 13.0 ± 0.2 | CB2 (220x) |  |
| JWH-360 [14] | Dibenzopyran | 2449 ± 606 | 160 ± 8 | CB2 (15x) |  |
| JWH-361 [14] | Dibenzopyran | 63 ± 3 | 2.7 ± 0.1 | CB2 (23x) |  |
| JWH-362 [14] | Dibenzopyran | 127 ± 8 | 34 ± 5 | CB2 (3.7x) |  |
| JWH-363 [13] | Naphthoylpyrrole | 245 ± 5 | 71 ± 1 | CB2 (3.45x) |  |
| JWH-364 [13] | Naphthoylpyrrole | 34 ± 3 | 29 ± 1 | CB2 (1.17x) |  |
| JWH-365 [13] | Naphthoylpyrrole | 17 ± 1 | 3.4 ± 0.2 | CB2 (5.0x) |  |
| JWH-366 [13] | Naphthoylpyrrole | 191 ± 12 | 24 ± 1 | CB2 (7.96x) |  |
| JWH-367 [13] | Naphthoylpyrrole | 53 ± 2 | 23 ± 1 | CB2 (2.30x) |  |
| JWH-368 [13] | Naphthoylpyrrole | 16 ± 1 | 9.1 ± 0.7 | CB2 (1.76x) | 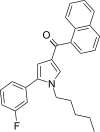 |
| JWH-369 [13] | Naphthoylpyrrole | 7.9 ± 0.4 | 5.2 ± 0.3 | CB2 (1.52x) |  |
| JWH-370 [13] | Naphthoylpyrrole | 5.6 ± 0.4 | 4.0 ± 0.5 | CB2 (1.40x) |  |
| JWH-371 [13] | Naphthoylpyrrole | 42 ± 1 | 64 ± 2 | CB1 (1.52x) | 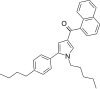 |
| JWH-372 [13] | Naphthoylpyrrole | 77 ± 2 | 8.2 ± 0.2 | CB1 (9.39x) |  |
| JWH-373 [13] | Naphthoylpyrrole | 60 ± 3 | 69 ± 2 | CB1 (1.15x) | 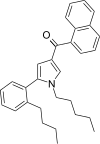 |
| JWH-387 [17] | Naphthoylindole | 1.2 ± 0.1 | 1.1 ± 0.1 | — |  |
| JWH-398 [18] | Naphthoylindole | 2.3 ± 0.1 | 2.8 ± 0.2 | CB1 (1.22x) | |
| JWH-416 [17] | Naphthoylindole | 73 ± 10 | 3.3 ± 0.1 | CB2 (22x) |  |
| JWH-417 [17] | Naphthoylindole | 522 ± 58 | 13 ± 0.2 | CB2 (40x) | 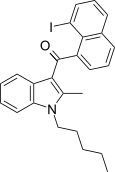 |
| JWH-422 [17] | Naphthoylindole | 501 ± 48 | 20 ± 0.4 | CB2 (25x) |  |
| JWH-423 [17] | Naphthoylindole | 140 ± 10 | 6.6 ± 0.2 | CB2 (21x) |  |
| JWH-424 [17] | Naphthoylindole | 21 ± 3.4 | 5.4 ± 0.2 | CB2 (3.9x) |  |
| JWH-425 [17] | Naphthoylindole | 54 ± 11 | 10 ± 0.4 | CB2 (5.4x) |  |