
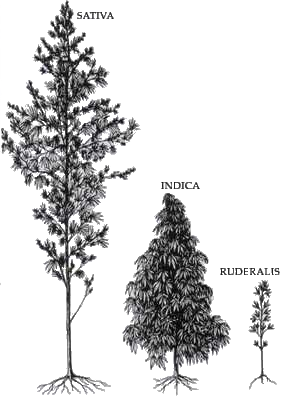
Cannabis is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: Cannabis sativa, C. indica, and C. ruderalis. Alternatively, C. ruderalis may be included within C. sativa, or all three may be treated as subspecies of C. sativa, or C. sativa may be accepted as a single undivided species. The genus is widely accepted as being indigenous to and originating from Asia.
Tetrahydrocannabinol (THC) is a cannabinoid found in cannabis. It is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. It is a colorless oil.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabis sativa is an annual herbaceous flowering plant. The species was first classified by Carl Linnaeus in 1753. The specific epithet sativa means 'cultivated'. Indigenous to Eastern Asia, the plant is now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history and used as a source of industrial fiber, seed oil, food, and medicine. It is also used as a recreational drug and for religious and spiritual purposes.
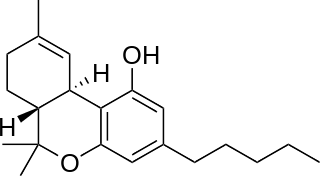
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Cannabivarin (CBV), also known as cannabivarol, is considered a non-psychoactive cannabinoid — it does not produce the euphoric side effects found in THC. Minor amounts of CBV are found in the hemp plant Cannabis sativa. It is an analog of cannabinol (CBN) with the side chain shortened by two methylene bridges (-CH2-). CBV is an oxidation product of tetrahydrocannabivarin (THCV, THV).

Cannabidivarin (CBDV, GWP42006) is a non-intoxicating psychoactive cannabinoid found in Cannabis. It is a homolog (chemistry) of cannabidiol (CBD), with the side-chain shortened by two methylene bridges (CH2 units).

Cannabis tea is a cannabis-infused drink prepared by steeping various parts of the cannabis plant in hot or cold water. Cannabis tea is commonly recognized as an alternative form of preparation and consumption of the cannabis plant, more popularly known as marijuana, pot, or weed. This plant has long been recognized as an herbal medicine employed by health professionals worldwide to ease symptoms of disease, as well as a psychoactive drug used recreationally and in spiritual traditions. Though less commonly practiced than popular methods like smoking or consuming edibles, drinking cannabis tea can produce comparable physical and mental therapeutic effects. Such effects are largely attributed to the THC and CBD content of the tea, levels of which are drastically dependent on individual preparation techniques involving volume, amount of cannabis, and boiling time. Also in common with these administration forms of cannabis is the heating component performed before usage. Due to the rather uncommon nature of this particular practice of cannabis consumption in modern times as well as the legality of cannabis throughout the world, the research available on the composition of cannabis tea is limited and based broadly around what is known of cannabis as it exists botanically.

Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.

Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, exhibits anti-inflammatory properties in vitro, which may, theoretically, contribute to cannabis analgesic effects. It is a phytocannabinoid, one of the hundreds of cannabinoids found in the Cannabis plant. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and cannabinols are present in cannabis. It is not scheduled by the Convention on Psychotropic Substances.

Cannabis strains is a popular name to refer to plant varieties of the monospecific genus Cannabis sativa L.. They are either pure or hybrid varieties of the plant, which encompasses various sub-species C. sativa, C. indica, and C. ruderalis.

Dronabinol, sold under the brand names Marinol and Syndros, is the generic name for the molecule of (−)-trans-Δ9-tetrahydrocannabinol (THC) in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the U.S. Food and Drug Administration (FDA) as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting.

Tetrahydrocannabinolic acid (THCA) synthase is an enzyme responsible for catalyzing the formation of THCA from cannabigerolic acid (CBGA). THCA is the direct precursor of tetrahydrocannabinol (THC), the principal psychoactive component of cannabis, which is produced from various strains of Cannabis sativa. Therefore, THCA synthase is considered to be a key enzyme controlling cannabis psychoactivity. Polymorphisms of THCA synthase result in varying levels of THC in Cannabis plants, resulting in "drug-type" and "fiber-type" C. sativa varieties.
The entourage effect is a hypothesis that cannabis compounds other than tetrahydrocannabinol (THC) act synergistically with it to modulate the overall psychoactive effects of the plant.
The following outline is provided as an overview of and topical guide to the plant Cannabis sativa and its relatives Cannabis indica and Cannabis ruderalis, the drug cannabis (drug) and the industrial product hemp.
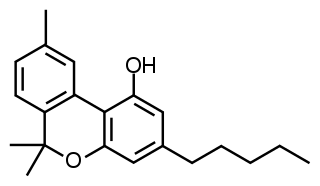
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by firstly acid cyclization of cannabidiol and then hydrogenation of tetrahydrocannabinol. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams.

Cannabimovone (CBM) is a phytocannabinoid first isolated from a non-psychoactive strain of Cannabis sativa in 2010, which is thought to be a rearrangement product of cannabidiol. It lacks affinity for cannabinoid receptors, but acts as an agonist at both TRPV1 and PPARγ.
Cannabinoids are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is another major constituent of some cannabis plants. Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.

















