
Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.
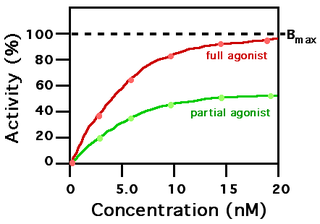
In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonistic effects—when both a full agonist and partial agonist are present, the partial agonist actually acts as a competitive antagonist, competing with the full agonist for receptor occupancy and producing a net decrease in the receptor activation observed with the full agonist alone. Clinically, partial agonists can be used to activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of taste, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.
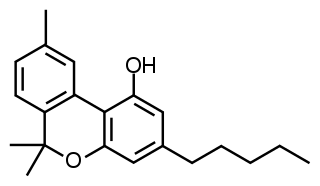
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

N-Arachidonyl glycine receptor, also known as G protein-coupled receptor 18 (GPR18), is a protein that in humans is encoded by the GPR18 gene. Along with the other previously orphan receptors GPR55 and GPR119, GPR18 has been found to be a receptor for endogenous lipid neurotransmitters, several of which also bind to cannabinoid receptors. It has been found to be involved in the regulation of intraocular pressure.

G protein-coupled receptor 55 also known as GPR55 is a G protein-coupled receptor that in humans is encoded by the GPR55 gene.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activate the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol.

G-protein coupled receptor 3 is a protein that in humans is encoded by the GPR3 gene. The protein encoded by this gene is a member of the G protein-coupled receptor family of transmembrane receptors and is involved in signal transduction.

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive or sedative effects. Abn-CBD can be found as an impurity in synthetic cannabidiol.

Tetrahydrocannabinolic acid is a precursor of tetrahydrocannabinol (THC), an active component of cannabis.

O-1602 is a synthetic compound most closely related to abnormal cannabidiol, and more distantly related in structure to cannabinoid drugs such as THC. O-1602 does not bind to the classical cannabinoid receptors CB1 or CB2 with any significant affinity, but instead is an agonist at several other receptors which appear to be related to the cannabinoid receptors, particularly GPR18 and GPR55. These previously orphan receptors have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research. O-1602 produces some effects shared with classical cannabinoid compounds such as analgesic and antiinflammatory effects and appetite stimulation, but it does not produce sedation or psychoactive effects, and has several actions in the gut and brain that are not shared with typical cannabinoid agonists.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.
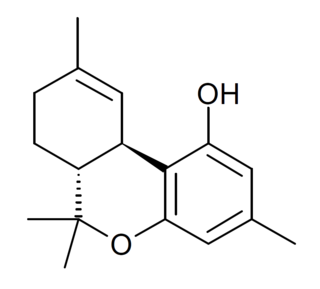
Δ9-Tetrahydrocannabiorcol (Δ9-THCC, (C1)-Δ9-THC) is a phytocannabinoid found in Cannabis pollen. It is a homologue of THC and THCV with the alkyl side chain replaced by a smaller methyl group. Unlike THC and THCV, THCC has negligible affinity for the CB1 and CB2 cannabinoid receptors because of the smaller methyl group and does not have psychoactive effects as a result, but conversely it is significantly more potent than THC or THCV as an activator of the TRPA1 calcium channel which plays an important role in pain perception, and it has been shown to produce analgesic effects via activation of spinal TRPA1 channels. THCC was studied by Roger Adams as early as 1942.

Tetrahydrocannabihexol is a phytocannabinoid, the hexyl homologue of tetrahydrocannabinol (THC) which was first isolated from Cannabis plant material in 2020 along with the corresponding hexyl homologue of cannabidiol, though it had been known for several decades prior to this as an isomer of the synthetic cannabinoid parahexyl. Another isomer Δ8-THCH is also known as a synthetic cannabinoid under the code number JWH-124, though it is unclear whether this occurs naturally in Cannabis, but likely is due to Δ8-THC itself being a degraded form of Δ9-THC. THC-Hexyl can be synthesized from 4-hexylresorcinol and was studied by Roger Adams as early as 1942.

Cannabimovone (CBM) is a phytocannabinoid first isolated from a non-psychoactive strain of Cannabis sativa in 2010, which is thought to be a rearrangement product of cannabidiol. It lacks affinity for cannabinoid receptors, but acts as an agonist at both TRPV1 and PPARγ.



















