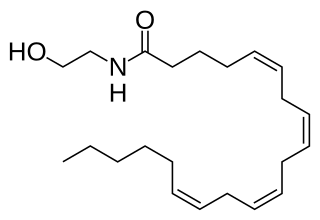
Anandamide (ANA), also referred to as N-arachidonoylethanolamine (AEA) is a fatty acid neurotransmitter belonging to the fatty acid derivative group known as N-acylethanolamine (NAE). Anandamide takes its name from the Sanskrit word ananda, meaning "joy, bliss, delight," plus amide. Anandamide, the first discovered endocannabinoid, engages with the body's endocannabinoid system by binding to the same cannabinoid receptors that THC found in cannabis acts on. Anandamide can be found within tissues in a wide range of animals. It has also been found in plants, such as the cacao tree.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands:

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the central nervous system and peripheral nervous system. The endocannabinoid system is still not fully understood, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

Fatty-acid amide hydrolase 1 (FAAH) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide (AEA), an N-acylethanolamine (NAE) in 1993. In humans, it is encoded by the gene FAAH.

2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor. It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994–1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid. AM404 is one of the AM cannabinoids discovered by Alexandros Makriyannis and his team.

Methoxy arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases. It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM), but does not appear to agonize or antagonize the receptor, though some related derivatives do show cannabinoid-like properties.

Virodhamine (O-arachidonoyl ethanolamine; O-AEA) is an endocannabinoid and a nonclassic eicosanoid, derived from arachidonic acid. O-Arachidonoyl ethanolamine is arachidonic acid and ethanolamine joined by an ester linkage, the opposite of the amide linkage found in anandamide. Based on this opposite orientation, the molecule was named virodhamine from the Sanskrit word virodha, which means opposition. It acts as an antagonist of the CB1 receptor and agonist of the CB2 receptor. Concentrations of virodhamine in the human hippocampus are similar to those of anandamide, but they are 2- to 9-fold higher in peripheral tissues that express CB2. Virodhamine lowers body temperature in mice, demonstrating cannabinoid activity in vivo.

N-Arachidonyl glycine receptor, also known as G protein-coupled receptor 18 (GPR18), is a protein that in humans is encoded by the GPR18 gene. Along with the other previously orphan receptors GPR55 and GPR119, GPR18 has been found to be a receptor for endogenous lipid neurotransmitters, several of which also bind to cannabinoid receptors. It has been found to be involved in the regulation of intraocular pressure.
The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator.

O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a Ki of 3.4 nM at CB1 and 3870 nM at CB2. Unlike most related compounds, O-1812 is metabolically stable against rapid breakdown by enzymes, and produces a cannabinoid-like discriminative effect in rats, which is similar but not identical to that produced by cannabinoid drugs of other chemical classes.

LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.

N-Arachidonylglycine (NAGly) is a carboxylic metabolite of the endocannabinoid anandamide (AEA). Since it was first synthesized in 1996, NAGly has been a primary focus of the relatively contemporary field of lipidomics due to its wide range of signaling targets in the brain, the immune system and throughout various other bodily systems. In combination with 2‐arachidonoyl glycerol (2‐AG), NAGly has enabled the identification of a family of lipids often referred to as endocannabinoids. Recently, NAGly has been found to bind to G-protein coupled receptor 18 (GPR18), the putative abnormal cannabidiol receptor. NaGly is an endogenous inhibitor of fatty acid amide hydrolase (FAAH) and thereby increases the ethanolamide endocannabinoids AEA, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels. NaGly is found throughout the body and research on its explicit functions is ongoing.

Serinolamide A is a naturally occurring eicosanoid derivative related to anandamide, which has been isolated from the marine cyanobacteria Lyngbya majuscula and related species in the Oscillatoria family.
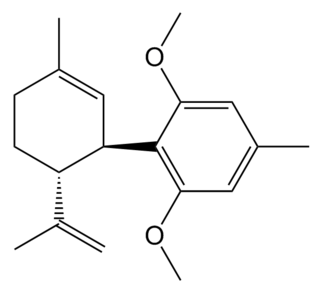
O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan receptors GPR18 and GPR55, that appear to be related to the cannabinoid receptors. O-1918 is used in the study of these receptors, which have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.
Daniele Piomelli is an Italian-born American scientist. He studied neuroscience in New York City, with James H. Schwartz and Eric R. Kandel at Columbia University College of Physicians and Surgeons and later with Paul Greengard at the Rockefeller University. Two of his mentors received in 2000 the Nobel Prize for their contributions to medicine. After working at the INSERM in Paris (1990–1995) and at the Neurosciences Institute in La Jolla (1995–1998) with Nobel Prize winner Gerald Edelman, he joined the University of California Irvine School of Medicine, where he is now Louise Turner Arnold Chair in Neurosciences and Professor of Anatomy and Neurobiology, Pharmacology and Biological Chemistry. He is also founding director of the department of Drug Discovery and Development (D3) at the Istituto Italiano di Tecnologia in Genova, Italy. He is also the editor of Cannabis and Cannabinoid Research and a board member of the non-profit International Association for Cannabinoid Medicines.

Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative.

















