
JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.

NESS-0327 is a drug used in scientific research which acts as an extremely potent and selective antagonist of the cannabinoid receptor CB1. It is much more potent an antagonist, and more selective for the CB1 receptor over CB2, than the more commonly used ligand rimonabant, with a Ki at CB1 of 350fM (i.e. 0.00035nM) and a selectivity of over 60,000x for CB1 over CB2. Independently, two other groups have described only modest nanomolar CB1 affinity for this compound (125nM and 18.4nM). Also unlike rimonabant, NESS-0327 does not appear to act as an inverse agonist at higher doses, instead being a purely neutral antagonist which blocks the CB1 receptor but does not produce any physiological effect of its own.

AM-4030 is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-((E)-3-hydroxyprop-1-enyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-4030 represents a hybrid structure between the classical and nonclassical cannabinoid families, with the 6-hydroxyalkyl chain rigidified with a double bond with defined stereochemistry. This gives AM-4030 a greater degree of selectivity, so while it is still a potent agonist at both CB1 and CB2, it is reasonably selective for CB1, with a Ki of 0.7nM at CB1 and 8.6nM at CB2, a selectivity of around 12x. Resolution of the enantiomers of AM-4030 yields an even more potent compound, although with less selectivity, with the (−) enantiomer AM-4030a having a Ki of 0.6nM at CB1 and 1.1nM at CB2.

JTE-907 is a drug used in scientific research that acts as a selective CB2 inverse agonist. It has antiinflammatory effects in animal studies, thought to be mediated by an interaction between the CB2 receptor and IgE.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.

SER-601 (COR-167) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, based on a quinolone-3-carboxylic acid core structure, with 190 times selectivity for CB2 over the related CB1 receptor. It has analgesic effects in animal studies, as well as neuroprotective effects, but without a "cannabis high" due to its low affinity for CB1. A number of related compounds are known, almost all of which have high selectivity for CB2.

AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.
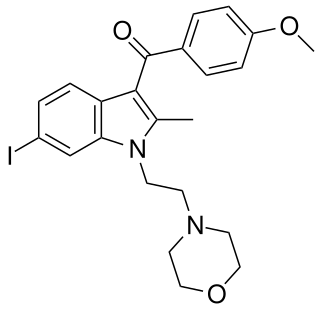
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a Ki of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (R) enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2.
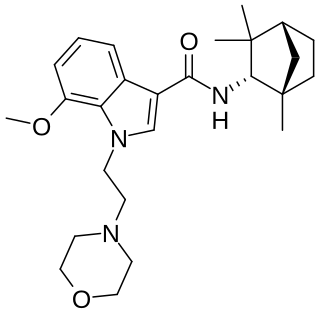
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories, that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor.

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.

AM-2389 is a classical cannabinoid derivative which acts as a potent and reasonably selective agonist for the CB1 receptor, with a Ki of 0.16 nM, and 26× selectivity over the related CB2 receptor. It has high potency in animal tests of cannabinoid activity, and a medium duration of action. Replacing the 1',1'-dimethyl substitution of the dimethylheptyl side chain of classical cannabinoids with cyclopropyl or cyclopentyl results in higher potency than cyclobutyl, but only the cyclobutyl derivatives show selectivity for CB1 over CB2. High selectivity for CB1 over CB2 is difficult to achieve (cf. AM-906, AM-1235), as almost all commonly used CB1 agonists have similar or greater affinity for CB2 than CB1, and the only truly highly selective CB1 agonists known as of 2012 are eicosanoid derivatives such as O-1812.

KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor. In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor. Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.

QMPSB is an arylsulfonamide-based synthetic cannabinoid that has been sold as a designer drug.

ADB-HEXINACA is a cannabinoid designer drug that has been found as an ingredient in some synthetic cannabis products, first appearing in early 2021. It is a longer chain homologue of previously encountered synthetic cannabinoid compounds such as ADB-BUTINACA and ADB-PINACA. The pharmacology of ADB-HEXINACA and numerous analogues at CB1 and CB2 receptors has been reported.
















