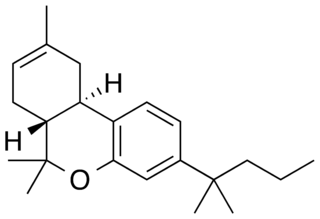
JWH-133(Dimethylbutyl-deoxy-Delta-8-THC) is a potent selective CB2 receptor agonist with a Ki of 3.4nM and selectivity of around 200x for CB2 over CB1 receptors. It was discovered by and named after, John W. Huffman.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.
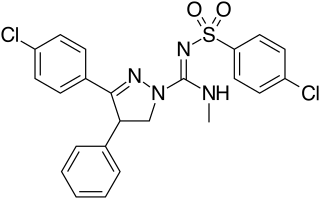
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist. It has potent anorectic effects in animals, and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists. SLV330, which is a structural analogue of Ibipinabant, was reported active in animal models related to the regulation of memory, cognition, as well as in addictive behavior. An atom-efficient synthesis of ibipinabant has been reported.

JWH-007 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It was first reported in 1994 by a group including the noted cannabinoid chemist John W. Huffman. It was the most active of the first group of N-alkyl naphoylindoles discovered by the team led by John W Huffman, several years after the family was initially described with the discovery of the N-morpholinylethyl compounds pravadoline (WIN 48,098), JWH-200 (WIN 55,225) and WIN 55,212-2 by the Sterling Winthrop group. Several other N-alkyl substituents were found to be active by Huffman's team including the n-butyl, n-hexyl, 2-heptyl, and cyclohexylethyl groups, but it was subsequently determined that the 2-methyl group on the indole ring is not required for CB1 binding, and tends to increase affinity for CB2 instead. Consequently, the 2-desmethyl derivative of JWH-007, JWH-018, has slightly higher binding affinity for CB1, with an optimum binding of 9.00 nM at CB1 and 2.94 nM at CB2, and JWH-007 displayed optimum binding of 9.50 nM at CB1 and 2.94 nM at CB2.

SER-601 (COR-167) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, based on a quinolone-3-carboxylic acid core structure, with 190 times selectivity for CB2 over the related CB1 receptor. It has analgesic effects in animal studies, as well as neuroprotective effects, but without a "cannabis high" due to its low affinity for CB1. A number of related compounds are known, almost all of which have high selectivity for CB2.
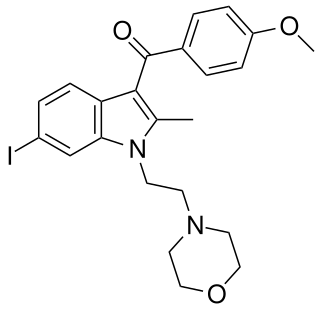
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.
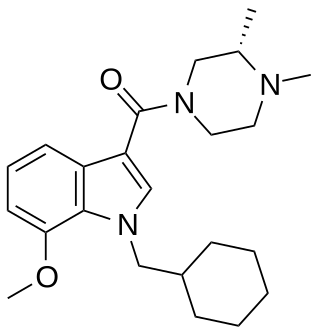
Org 28611 (SCH-900,111) is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, and while it achieved this aim and has progressed as far as Phase II clinical trials in humans as both a sedative and an analgesic, results against the comparison drugs (midazolam and morphine respectively) were not particularly favourable in initial testing.

AM-1220 is a drug that acts as a potent and moderately selective agonist for the cannabinoid receptor CB1, with around 19 times selectivity for CB1 over the related CB2 receptor. It was originally invented in the early 1990s by a team led by Thomas D'Ambra at Sterling Winthrop, but has subsequently been researched by many others, most notably the team led by Alexandros Makriyannis at the University of Connecticut. The (piperidin-2-yl)methyl side chain of AM-1220 contains a stereocenter, so there are two enantiomers with quite different potency, the (R)-enantiomer having a Ki of 0.27 nM at CB1 while the (S)-enantiomer has a much weaker Ki of 217 nM.
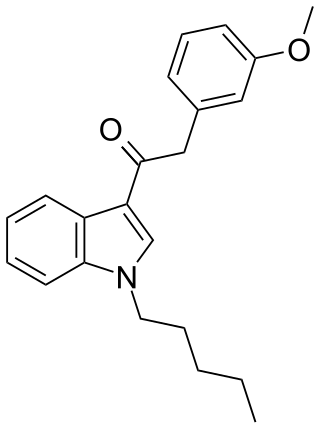
JWH-302 (1-pentyl-3-(3-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family, which acts as a cannabinoid agonist with moderate affinity at both the CB1 and CB2 receptors. It is a positional isomer of the more common drug JWH-250, though it is slightly less potent with a Ki of 17 nM at CB1, compared to 11 nM for JWH-250. Because of their identical molecular weight and similar fragmentation patterns, JWH-302 and JWH-250 can be very difficult to distinguish by GC-MS testing.
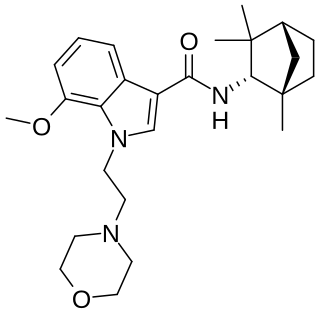
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.
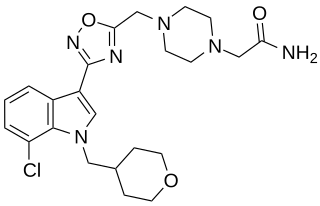
LBP-1 is a drug originally developed by Organon for the treatment of neuropathic pain, It acts as a potent and selective cannabinoid receptor agonist, with high potency at both the CB1 and CB2 receptors, but low penetration of the blood–brain barrier. This makes LBP-1 peripherally selective, and while it was effective in animal models of neuropathic pain and allodynia, it did not produce cannabinoid-appropriate responding suggestive of central effects, at any dose tested.

KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor. In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor. Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.
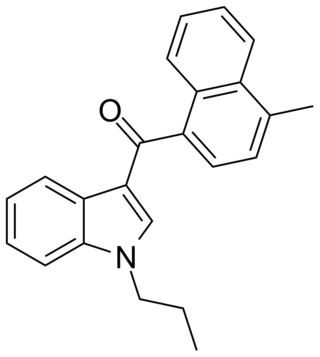
JWH-120 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-propyl analog of JWH-122. It is a potent and selective ligand for the CB2 receptor, but a weaker ligand for the CB1 receptor. It has a binding affinity of Ki = 6.1 ± 0.7 nM at the CB2 subtype and 173 times selectivity over the CB1 subtype.
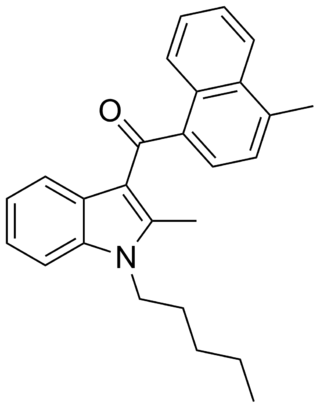
JWH-149 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-pentyl analog of JWH-148. It is a potent but only moderately selective ligand for the CB2 receptor, with a binding affinity of Ki = 0.73 ± 0.03 nM at this subtype, and more than six times selectivity over the CB1 subtype.

PTI-2 (SGT-49) is an indole-based synthetic cannabinoid. It is one of few synthetic cannabinoids containing a thiazole group and is closely related to PTI-1. These compounds may be viewed as simplified analogues of indole-3-heterocycle compounds originally developed by Organon and subsequently further researched by Merck.

PTI-1 (SGT-48) is an indole-based synthetic cannabinoid. It is one of few synthetic cannabinoids containing a thiazole group and is closely related to PTI-2. These compounds may be viewed as simplified analogues of indole-3-heterocycle compounds originally developed by Organon and subsequently further researched by Merck.

ADB-FUBHQUCA is a synthetic cannabinoid receptor agonist that has been sold as a designer drug, first reported in 2022. It is related to the previously reported compound ADB-FUBICA but with the central indole ring system expanded to a 1,4-dihydroquinoline structure. This breaks the aromaticity of the ring system, and ADB-FUBHQUCA is relatively low in potency compared to related compounds where the aromatic core is retained.

















