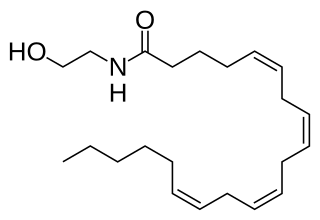
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), an N-acylethanolamine (NAE), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; phytocannabinoids ; and synthetic cannabinoids. All endocannabinoids and phytocannabinoids are lipophilic.
The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors (CBRs), and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

Fatty-acid amide hydrolase 1 or FAAH-1(EC 3.5.1.99, oleamide hydrolase, anandamide amidohydrolase) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide (AEA), an N-acylethanolamine (NAE) in 1993. In humans, it is encoded by the gene FAAH. FAAH also regulate the contents of NAE's in Dictyostelium discoideum, as they modulate their NAE levels in vivo through the use of a semispecific FAAH inhibitor.

2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor. It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994–1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. And a receptor being clearly present in bacteria, the oldest organisms on Earth known to express phosphatidylethanolamine, the precursor to endocannabinoids, in their cytoplasmic membranes, and fatty acid metabolites with affinity for this CB receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA).

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

N-Arachidonyl glycine receptor, also known as G protein-coupled receptor 18 (GPR18), is a protein that in humans is encoded by the GPR18 gene. Along with the other previously "orphan" receptors GPR55 and GPR119, GPR18 has been found to be a receptor for endogenous lipid neurotransmitters, several of which also bind to cannabinoid receptors. It has been found to be involved in the regulation of intraocular pressure.

G protein-coupled receptor 55 also known as GPR55 is a G protein-coupled receptor that in humans is encoded by the GPR55 gene.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

G protein-coupled receptor 119 also known as GPR119 is a G protein-coupled receptor that in humans is encoded by the GPR119 gene.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.

Oleoylethanolamide (OEA) is an endogenous peroxisome proliferator-activated receptor alpha (PPAR-α) agonist. It is a naturally occurring ethanolamide lipid that regulates feeding and body weight in vertebrates ranging from mice to pythons.

An N-acylethanolamine (NAE) is a type of fatty acid amide where one of several types of acyl groups is linked to the nitrogen atom of ethanolamine, and highly metabolic formed by intake of essential fatty acids through diet by 20:4, n-6 and 22:6, n-3 fatty acids, and when the body is physically and psychologically active,. The endocannabinoid signaling system (ECS) is the major pathway by which NAEs exerts its physiological effects in animal cells with similarities in plants, and the metabolism of NAEs is an integral part of the ECS, a very ancient signaling system, being clearly present from the divergence of the protostomian/deuterostomian, and even further back in time, to the very beginning of bacteria, the oldest organisms on Earth known to express phosphatidylethanolamine, the precursor to endocannabinoids, in their cytoplasmic membranes. Fatty acid metabolites with affinity for CB receptors are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA), by brown algae which diverged about 1500 MYA, by sponges, which diverged from eumetazoans about 930 MYA, and a lineages that predate the evolution of CB receptors, as CB1 – CB2 duplication event may have occurred prior to the lophotrochozoan-deuterostome divergence 590 MYA. Fatty acid amide hydrolase (FAAH) evolved relatively recently, either after the evolution of fish 400 MYA, or after the appearance of mammals 300 MYA, but after the appearance of vertebrates. Linking FAAH, vanilloid receptors (VR1) and anandamide implies a coupling among the remaining ‘‘older’’ parts of the endocannabinoid system, monoglyceride lipase (MGL), CB receptors, that evolved prior to the metazoan-bilaterian divergence, but were secondarily lost in the Ecdysozoa, and 2-Arachidonoylglycerol (2-AG).
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive effects.
RVD-Hpα (pepcan-12) is an endogenous neuropeptide found in human and mammalian brain, which was originally proposed to act as a selective agonist for the CB1 cannabinoid receptor. It is a 12-amino acid polypeptide having the amino acid sequence Arg-Val-Asp-Pro-Val-Asn-Phe-Lys-Leu-Leu-Ser-His and is an N-terminal extended form of hemopressin, a 9-AA polypeptide derived from the α1 subunit of hemoglobin which has previously been shown to act as a CB1 inverse agonist. All three polypeptides have been isolated from various mammalian species, with RVD-Hpα being one of the more abundant neuropeptides expressed in mouse brain, and these neuropeptides represent a new avenue for cannabinoid research distinct from the previously known endogenous lipid-derived cannabinoid agonists such as anandamide. Recently it was shown that RVD-Hpα (also called Pepcan-12) is a potent negative allosteric modulator at CB1 receptors, together with other newly described N-terminally extended peptides (pepcans).
Endocannabinoid reuptake inhibitors (eCBRIs), also called cannabinoid reuptake inhibitors (CBRIs), are drugs which limit the reabsorption of endocannabinoid neurotransmitters by the releasing neuron.
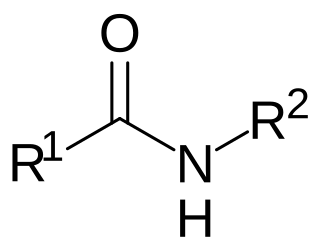
N-acyl amides are a general class of endogenous fatty acid compounds characterized by a fatty acyl group linked to a primary amine metabolite by an amide bond. Broadly speaking, N-acyl amides fall into several categories: amino acid conjugates, neurotransmitter conjugates, ethanolamine conjugates, and taurine conjugates. N-acyl amides have pleiotropic signaling functions in physiology, including in cardiovascular function, metabolic homeostasis, memory, cognition, pain, motor control and others. Initial attention focused on N-acyl amides present in mammalian organisms, however recently lipid signaling systems consisting of N-acyl amides have also been found to be present in invertebrates, such as Drosophila melanogaster. N-acyl amides play important roles in many biochemical pathways involved in a variety of physiological and pathological processes, as well as the metabolic enzymes, transporters, and receptors that regulate their signaling.
















