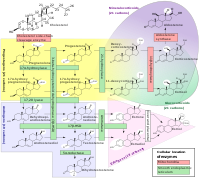
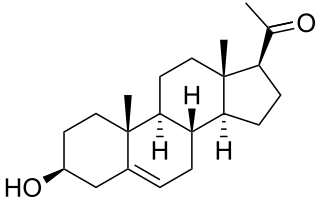
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.

Androstenol, also known as 5α-androst-16-en-3α-ol, is a 16-androstene class steroidal pheromone and neurosteroid in humans and other mammals, notably pigs. It possesses a characteristic musk-like odor.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Prasterone sulfate, also known as dehydroepiandrosterone sulfate (DHEA-S), is a naturally occurring androstane steroid which is marketed and used in Japan and other countries as a labor inducer in the treatment of insufficient cervical ripening and dilation during childbirth. It is the C3β sulfate ester of prasterone, and is known to act as a prohormone of DHEA and by extension of androgens and estrogens, although it also has its own activity as a neurosteroid. Prasterone sulfate is used medically as the sodium salt via injection and is referred to by the name sodium prasterone sulfate (JAN).

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
A neurosteroidogenesis inhibitor is a drug that inhibits the production of endogenous neurosteroids. Neurosteroids include the excitatory neurosteroids pregnenolone sulfate, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S), and the inhibitory neurosteroids allopregnanolone, tetrahydrodeoxycorticosterone (THDOC), and 3α-androstanediol, among others. By inhibiting the synthesis of endogenous neurosteroids, neurosteroidogenesis inhibitors have effects in the central nervous system.

Epipregnanolone, also known as 3β-hydroxy-5β-pregnan-20-one, 3β,5β-tetrahydroprogesterone, or 3β,5β-THP, is an endogenous neurosteroid. It acts as a negative allosteric modulator of the GABAA receptor and reverses the effects of potentiators like allopregnanolone. Epipregnanolone is biosynthesized from progesterone by the actions of 5β-reductase and 3β-hydroxysteroid dehydrogenase, with 5β-dihydroprogesterone as the intermediate in this two-step transformation.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

7β-Hydroxyepiandrosterone (7β-OH-EPIA), also known as 5α-androstan-3β,7β-diol-17-one, is an endogenous androgen, estrogen, and neurosteroid that is produced from dehydroepiandrosterone and epiandrosterone. It has neuroprotective effects and, along with 7α-hydroxyepiandrosterone, may mediate the neuroprotective effects of DHEA. 7β-OH-EPIA may act as a highly potent antagonist of the G protein-coupled estrogen receptor (GPER).

Pregnenolone succinate is a synthetic pregnane steroid and an ester of pregnenolone which is described as a glucocorticoid and anti-inflammatory drug and has been patented and marketed as a topical medication in the form of a cream for the treatment of allergic, pruritic, and inflammatory dermatitis. It has also been described as a non-hormonal sterol, having neurosteroid activity, and forming a progesterone analogue via dehydrogenation.

BNN-20, also known as 17β-spiro-(androst-5-en-17,2'-oxiran)-3β-ol, is a synthetic neurosteroid, "microneurotrophin", and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA, TrkB, and p75NTR, receptors for the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), as well as for DHEA and DHEA sulfate (DHEA-S). The drug has been suggested as a potential novel treatment for Parkinson's disease and other conditions.

BNN-27, also known as 17α,20R-epoxypregn-5-ene-3β,21-diol, is a synthetic neurosteroid and "microneurotrophin" and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA and p75NTR, receptors for nerve growth factor (NGF) and other neurotrophins, as well as for DHEA and DHEA sulfate (DHEA-S). BNN-27 has neuroprotective and neurogenic effects and has been suggested as a potential novel treatment for neurodegenerative diseases and brain trauma.

Steroid sulfates are endogenous sulfate esters of steroids. They are formed by steroid sulfotransferases via sulfation of endogenous steroids like cholesterol and steroid hormones. Although steroid sulfates do not bind to steroid hormone receptors and hence are hormonally inert, they can be desulfated by steroid sulfatase and in this way serve as precursors and circulating reservoirs for their active unsulfated counterparts. In addition, some steroid sulfates have biological activity in their own right, for instance acting as neurosteroids and modulating ligand-gated ion channels such as the GABAA and NMDA receptors among other biological targets.
A GABAA receptor negative allosteric modulator is a negative allosteric modulator (NAM), or inhibitor, of the GABAA receptor, a ligand-gated ion channel of the major inhibitory neurotransmitter γ-aminobutyric acid (GABA). They are closely related and similar to GABAA receptor antagonists. The effects of GABAA receptor NAMs are functionally the opposite of those of GABAA receptor positive allosteric modulators (PAMs) like the benzodiazepines, barbiturates, and ethanol (alcohol). Non-selective GABAA receptor NAMs can produce a variety of effects including convulsions, neurotoxicity, and anxiety, among others.

Pregnenolone, sold under the brand name Enelone among others, is a medication and supplement as well as a naturally occurring and endogenous steroid. It is described as a neurosteroid and anti-inflammatory drug and was used in the treatment of rheumatoid arthritis and soft-tissue rheumatism in the 1950s but is no longer used today. Pregnenolone can be taken by mouth, as a topical medication, or by injection into muscle.