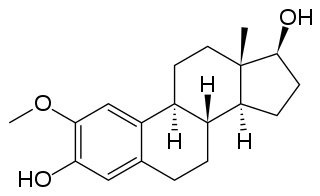
2-Methoxyestradiol is a natural metabolite of estradiol and 2-hydroxyestradiol (2-OHE2). It is specifically the 2-methyl ether of 2-hydroxyestradiol. 2-Methoxyestradiol prevents the formation of new blood vessels that tumors need in order to grow (angiogenesis), hence it is an angiogenesis inhibitor. It also acts as a vasodilator and induces apoptosis in some cancer cell lines. 2-Methoxyestradiol is derived from estradiol, although it interacts poorly with the estrogen receptors. However, it retains activity as a high-affinity agonist of the G protein-coupled estrogen receptor (GPER).

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Estrogen-related receptor gamma (ERR-gamma), also known as NR3B3, is a nuclear receptor that in humans is encoded by the ESRRG gene. It behaves as a constitutive activator of transcription.
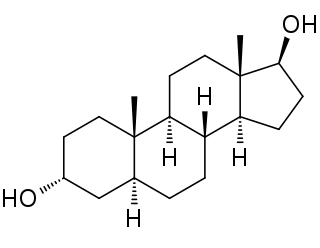
3α-Androstanediol also known as 5α-androstane-3α,17β-diol and sometimes shortened in the literature to 3α-diol, is an endogenous steroid hormone and neurosteroid and a metabolite of androgens like dihydrotestosterone (DHT).

Estetrol (E4), or oestetrol, is one of the four natural estrogenic steroid hormones found in humans, along with estrone (E1), estradiol (E2), and estriol (E3). Estetrol is a major estrogen in the body. In contrast to estrone and estradiol, estetrol is a native estrogen of fetal life. Estetrol is produced exclusively by the fetal liver and is found in detectable levels only during pregnancy, with relatively high levels in the fetus and lower levels in the maternal circulation.

Moxestrol, sold under the brand name Surestryl, is an estrogen medication which has been used in Europe for the treatment of menopausal symptoms and menstrual disorders. It is taken by mouth. In addition to its use as a medication, moxestrol has been used in scientific research as a radioligand of the estrogen receptor.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.

17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol. It is formed from 16α-hydroxyestrone. In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ. It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα. 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression in vitro.

2-Hydroxyestradiol (2-OHE2), also known as estra-1,3,5(10)-triene-2,3,17β-triol, is an endogenous steroid, catechol estrogen, and metabolite of estradiol, as well as a positional isomer of estriol.

A catechol estrogen is a steroidal estrogen that contains catechol (1,2-dihydroxybenzene) within its structure. The catechol estrogens are endogenous metabolites of estradiol and estrone and include the following compounds:

Estradiol glucuronide, or estradiol 17β-D-glucuronide, is a conjugated metabolite of estradiol. It is formed from estradiol in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estradiol. Glucuronides are the most abundant estrogen conjugates.

2-Hydroxyestrone (2-OHE1), also known as estra-1,3,5(10)-trien-2,3-diol-17-one, is an endogenous, naturally occurring catechol estrogen and a major metabolite of estrone and estradiol. It is formed irreversibly from estrone in the liver and to a lesser extent in other tissues via 2-hydroxylation mediated by cytochrome P450 enzymes, mainly the CYP3A and CYP1A subfamilies. 2-OHE1 is the most abundant catechol estrogen in the body.

2-Methoxyestrone (2-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 2-hydroxyestrone. Unlike estrone but similarly to 2-hydroxyestrone and 2-methoxyestradiol, 2-methoxyestrone has very low affinity for the estrogen receptor and lacks significant estrogenic activity.
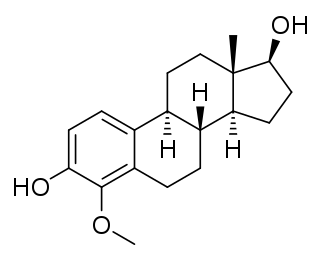
4-Methoxyestradiol (4-ME2) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estradiol that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestradiol. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

4-Methoxyestrone (4-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestrone. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

The hydroxylation of estradiol is one of the major routes of metabolism of the estrogen steroid hormone estradiol. It is hydroxylated into the catechol estrogens 2-hydroxyestradiol and 4-hydroxyestradiol and into estriol (16α-hydroxyestradiol), reactions which are catalyzed by cytochrome P450 enzymes predominantly in the liver, but also in various other tissues.

5α-Dihydrolevonorgestrel (5α-DHLNG) is an active metabolite of the progestin levonorgestrel which is formed by 5α-reductase. It has about one-third of the affinity of levonorgestrel for the progesterone receptor. In contrast to levonorgestrel, the compound has both progestogenic and antiprogestogenic activity, and hence has a selective progesterone receptor modulator-like profile of activity. This is analogous to the case of norethisterone and 5α-dihydronorethisterone. In addition to the progesterone receptor, 5α-DHLNG interacts with the androgen receptor. It has similar affinity for the androgen receptor relative to levonorgestrel, and has androgenic effects similarly to levonorgestrel and testosterone. 5α-DHLNG is further transformed into 3α,5α- and 3β,5α-THLNG, which bind weakly to the estrogen receptor and have weak estrogenic activity. These metabolites are considered to be responsible for the weak estrogenic activity of high doses of levonorgestrel.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.

4-Methoxyestriol (4-MeO-E3) is an endogenous estrogen metabolite. It is the 4-methyl ether of 4-hydroxyestriol and a metabolite of estriol and 4-hydroxyestriol. 4-Methoxyestriol has very low affinities for the estrogen receptors. Its relative binding affinities (RBAs) for estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) are both about 1% of those of estradiol. For comparison, estriol had RBAs of 11% and 35%, respectively.

11β-Chloromethylestradiol is a synthetic steroidal estrogen which was never marketed. It has very high affinity for the estrogen receptor and dissociates from it relatively slowly. It was originally thought that 11β-CME2 might be a covalent ligand of the estrogen receptors, but its binding was subsequently shown to be fully reversible. The relative binding affinity of 11β-CME2 for the estrogen receptors ranges from 230 to 3,320% of that of estradiol depending on the study. 11β-CME2 also has about 14% of the relative binding affinity of estradiol for sex hormone-binding globulin (SHBG). The compound has been developed as a radiolabel for the ERs.



















