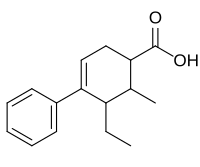
Emergency contraception (EC) is a birth control measure, used after sexual intercourse to prevent pregnancy.

Levonorgestrel is a hormonal medication which is used in a number of birth control methods. It is combined with an estrogen to make combination birth control pills. As an emergency birth control, sold under the brand names Plan B One-Step and Julie, among others, it is useful within 72 hours of unprotected sex. The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred. Levonorgestrel works by preventing ovulation or fertilization from occurring. It decreases the chances of pregnancy by 57 to 93%. In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy. A levonorgestrel-releasing implant is also available in some countries.
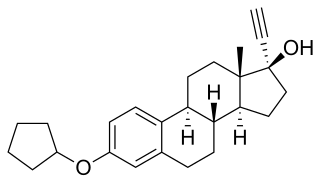
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer. It is taken once per week to once per month by mouth.
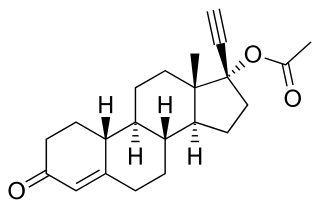
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Norgestrel is a progestin which is used in birth control pills sold under the brand name Ovral in combination with the estrogen ethinylestradiol and Opill by itself. It is also used in menopausal hormone therapy. It is taken by mouth.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Lynestrenol, sold under the brand names Exluton and Ministat among others, is a progestin medication which is used in birth control pills and in the treatment of gynecological disorders. The medication is available both alone and in combination with an estrogen. It is taken by mouth.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.

Zeranol, or zearanol, also known as α-zearalanol or simply zearalanol, is a synthetic nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in fungi in the Fusarium genus and is used mainly as an anabolic agent in veterinary medicine.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.

Nafoxidine or nafoxidine hydrochloride (USAN) is a nonsteroidal selective estrogen receptor modulator (SERM) or partial antiestrogen of the triphenylethylene group that was developed for the treatment of advanced breast cancer by Upjohn in the 1970s but was never marketed. It was developed at around the same time as tamoxifen and clomifene, which are also triphenylethylene derivatives. The drug was originally synthesized by the fertility control program at Upjohn as a postcoital contraceptive, but was subsequently repurposed for the treatment of breast cancer. Nafoxidine was assessed in clinical trials in the treatment of breast cancer and was found to be effective. However, it produced side effects including ichthyosis, partial hair loss, and phototoxicity of the skin in almost all patients, and this resulted in the discontinuation of its development.

Methallenestril (INN), also known as methallenoestril (BAN) and as methallenestrol, as well as Horeau's acid, is a synthetic nonsteroidal estrogen and a derivative of allenolic acid and allenestrol that was formerly used to treat menstrual issues but is now no longer marketed. It is a seco-analogue of bisdehydrodoisynolic acid, and although methallenestril is potently estrogenic in rats, in humans it is only weakly so in comparison. Vallestril was a brand of methallenestril issued by G. D. Searle & Company in the 1950s. Methallenestril is taken by mouth. By the oral route, a dose of 25 mg methallenestril is approximately equivalent to 1 mg diethylstilbestrol, 4 mg dienestrol, 20 mg hexestrol, 25 mg estrone, 2.5 mg conjugated estrogens, and 0.05 mg ethinylestradiol.
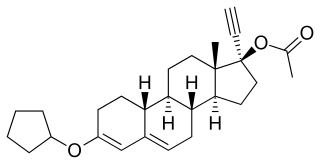
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Norgesterone, also known as norvinodrel or vinylestrenolone and sold under the brand name Vestalin, is a progestin medication which was formerly used in birth control pills for women but is now no longer marketed. It was used in combination with the estrogen ethinylestradiol. It is taken by mouth.
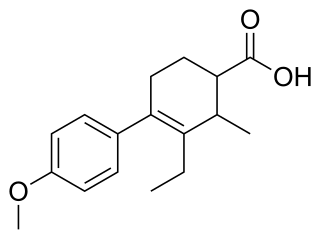
Carbestrol is a synthetic, nonsteroidal estrogen of the cyclohexenecarboxylic acid group and seco analogue of doisynolic acid that was described in the literature in 1956 and developed for the treatment of prostate cancer in the 1960s but was never marketed.

Anordrin, also known as 2α,17α-diethynyl-A-nor-5α-androstane-2β,17β-diol dipropionate, is a synthetic, steroidal selective estrogen receptor modulator (SERM) which is used in China as an emergency contraceptive. It is the most commonly used emergency contraceptive in China. The drug is marketed in a combination formulation with mifepristone under the brand name Zi Yun. Anordrin has not been studied for use or marketed outside of China. It has been used in China since the 1970s.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Doisynoestrol, also known as fenocycline, as well as cis-bisdehydrodoisynolic acid 7-methyl ether, is a synthetic nonsteroidal estrogen of the doisynolic acid group that is no longer marketed. It is a methyl ether of bisdehydrodoisynolic acid. Doisynoestrol was described in the literature in 1945. It has about 0.02% of the relative binding affinity of estradiol for the estrogen receptor.
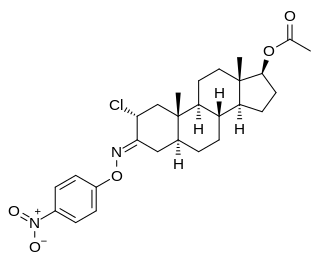
Nisterime acetate (USAN) (developmental code name ORF-9326), also known as 2α-chloro-4,5α-dihydrotestosterone O-(p-nitrophenyl)oxime 17β-acetate or as 2α-chloro-5α-androstan-17β-ol-3-one O-(p-nitrophenyl)oxime 17β-acetate, is a synthetic, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT) that was developed as a postcoital contraceptive but was never marketed. It is an androgen ester – specifically, the C17α acetate ester of nisterime. Unlike antiprogestogens like mifepristone, nisterime acetate does not prevent implantation and instead induces embryo resorption as well as interrupts the post-implantation stage of pregnancy.