
Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.
Burow's solution is an aqueous solution of aluminium triacetate. It is available in the U.S. as an over-the-counter drug for topical administration, with brand names including Domeboro, Domeboro Otic, Star-Otic, and Borofair. The preparation has astringent and antibacterial properties and may be used to treat a number of skin conditions, including insect bites and stings, rashes caused by poison ivy and poison sumac, swelling, allergies, and bruises. However, its main use is for treatment of otitis, including otomycosis.

Aspergillosis is a fungal infection of usually the lungs, caused by the genus Aspergillus, a common mould that is breathed in frequently from the air, but does not usually affect most people. It generally occurs in people with lung diseases such as asthma, cystic fibrosis or tuberculosis, or those who are immunocompromised such as those who have had a stem cell or organ transplant or those who take medications such as steroids and some cancer treatments which suppress the immune system. Rarely, it can affect skin.

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:

Dual specificity mitogen-activated protein kinase kinase 5 is an enzyme that in humans is encoded by the MAP2K5 gene.

Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes which can be formed by coupling of the B ring to the 4-one position.
Glyceollins are a family of prenylated pterocarpans found in ineffective types of nodule in soybean in response to symbiotic infection.
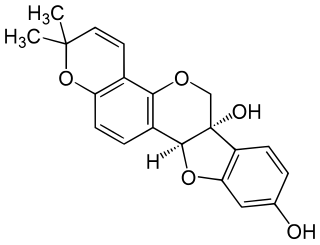
Glyceollin I is a glyceollin, a type of prenylated pterocarpan. It is a phytoalexin found in the soybean.

Glycinol is a pterocarpan, a type of natural phenol. It is a phytoalexin found in the soybean. It is formed by the cyclisation of daidzein.

Aspergillus sojae is a species of fungus in the genus Aspergillus.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Aspergillus austroafricanus is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 2012. It has been isolated in South Africa.
Aspergillus fructus is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 2012.
Aspergillus jensenii is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 2012.
Aspergillus puulaauensis is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 2012.
Aspergillus subversicolor is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 2012.

Aspergillus tennesseensis is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 2012.
Aspergillus tabacinus is a species of fungus in the genus Aspergillus. It is from the Versicolores section. The species was first described in 1934.
Kōji refers to various molds of the genus Aspergillus sp., which are traditionally used in East Asian cuisine for the fermentation of food. In Japanese, kōji refers to both the Aspergillus starter culture and mixtures of Aspergillus with wheat and soybean meal. It can be fried and eaten directly or processed to a sauce.









