Estrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy.

Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used. In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses. By 2007, it was only used in the treatment of prostate cancer and breast cancer. In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer. While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.

Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol.
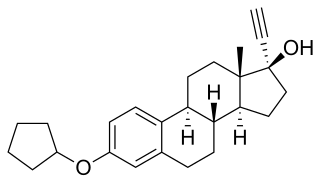
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer. It is taken once per week to once per month by mouth.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.

Methallenestril, also known as methallenoestril and as methallenestrol, as well as Horeau's acid, is a synthetic nonsteroidal estrogen and a derivative of allenolic acid and allenestrol that was formerly used to treat menstrual issues but is now no longer marketed. It is a seco-analogue of bisdehydrodoisynolic acid, and although methallenestril is potently estrogenic in rats, in humans it is only weakly so in comparison. Vallestril was a brand of methallenestril issued by G. D. Searle & Company in the 1950s. Methallenestril is taken by mouth. By the oral route, a dose of 25 mg methallenestril is approximately equivalent to 1 mg diethylstilbestrol, 4 mg dienestrol, 20 mg hexestrol, 25 mg estrone, 2.5 mg conjugated estrogens, and 0.05 mg ethinylestradiol.

Moxestrol, sold under the brand name Surestryl, is an estrogen medication which has been used in Europe for the treatment of menopausal symptoms and menstrual disorders. It is taken by mouth. In addition to its use as a medication, moxestrol has been used in scientific research as a radioligand of the estrogen receptor.
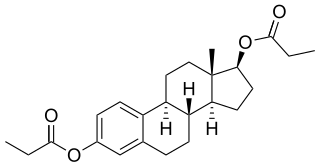
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the treatment of gynecological disorders. It has also been used in feminizing hormone therapy for transgender women and in the treatment of prostate cancer in men. Although widely used in the past, estradiol dipropionate has largely been discontinued and is mostly no longer available today. It appears to remain in use only in Japan, Macedonia, and Australia. Estradiol dipropionate is given by injection into muscle at intervals ranging from once or twice a week to once every week and a half to two weeks.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine for the treatment of enlarged prostate in dogs. It is given by mouth.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Diethylstilbestrol dipropionate (DESDP), or diethylstilbestrol dipropanoate, also known as stilboestrol dipropionate, is a synthetic nonsteroidal estrogen of the stilbestrol group that was formerly marketed widely throughout Europe. It is an ester of diethylstilbestrol with propionic acid, and is more slowly absorbed in the body than diethylstilbestrol. The medication has been said to be one of the most potent estrogens known.

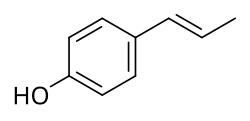
Dianethole is a naturally occurring organic compound that is found in anise and fennel. It is a dimeric polymer of anethole. It has estrogenic activity, and along with anethole and photoanethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after dianethole and photoanethole.

Dianol is a synthetic, nonsteroidal estrogen that was never marketed. It is a dimer and impurity of anol, and was, along with hexestrol, involved in erroneous findings of highly potent estrogenic activity with anol. Although a potent estrogen, it requires a dose of 100 μg to show activity, whereas hexestrol shows activity with a mere dose of 0.2 μg.
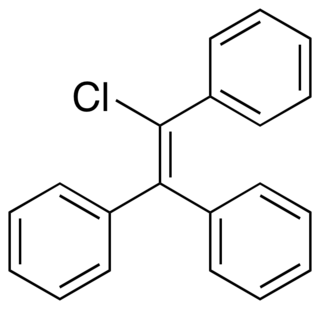
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

Photoanethole is a naturally occurring organic compound that is found in anise and fennel. It has estrogenic activity, and along with anethole and dianethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after photoanethole and dianethole.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.