 | |
| Clinical data | |
|---|---|
| Pronunciation | /fʊlˈvɛstrənt/ fuul-VES-trənt |
| Trade names | Faslodex, others |
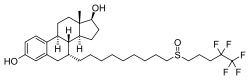
| Other names | ICI-182780; ZD-182780; ZD-9238; 7α-[9-[(4,4,5,5,5-Pentafluoropentyl)-sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607031 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular injection |
| Drug class | Antiestrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low [4] |
| Protein binding | 99% [4] |
| Metabolism | Hydroxylation, conjugation (glucuronidation, sulfation) [4] |
| Elimination half-life | IM : 40–50 days [4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.170.955 |
| Chemical and physical data | |
| Formula | C32H47F5O3S |
| Molar mass | 606.78 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fulvestrant, sold under the brand name Faslodex among others, is an antiestrogenic medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with abemaciclib or palbociclib in women with disease progression after endocrine therapy. [2] It is given by injection into a muscle. [5]
Contents
- Medical uses
- Breast cancer
- Early puberty
- Available forms
- Contraindications
- Side effects
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- Synthesis
- History
- Society and culture
- NICE evaluation
- Patent extension
- Research
- References
Fulvestrant is a selective estrogen receptor degrader (SERD) and was first-in-class to be approved. [6] It works by binding to the estrogen receptor and destabilizing it, causing the cell's normal protein degradation processes to destroy it. [6]
Fulvestrant was approved for medical use in the United States in 2002. [7]
