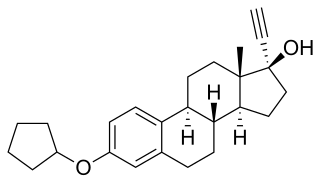
Quinestrol, also known as ethinylestradiol cyclopentyl ether (EECPE), sold under the brand name Estrovis among others, is an estrogen medication which has been used in menopausal hormone therapy, hormonal birth control, and to treat breast cancer and prostate cancer. It is taken once per week to once per month by mouth.

Estradiol acetate (EA), sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women. It is taken by mouth once daily or given as a vaginal ring once every three months.

Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. It is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender people, and in hormonal birth control. It is also used in the treatment of prostate cancer. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.

Polyestradiol phosphate (PEP), sold under the brand name Estradurin, is an estrogen medication which is used primarily in the treatment of prostate cancer in men. It is also used in women to treat breast cancer, as a component of hormone therapy to treat low estrogen levels and menopausal symptoms, and as a component of feminizing hormone therapy for transgender women. It is given by injection into muscle once every four weeks.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Dienestrol diacetate is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It is an ester of dienestrol.

Diethylstilbestrol dipropionate (DESDP), or diethylstilbestrol dipropanoate, also known as stilboestrol dipropionate, is a synthetic nonsteroidal estrogen of the stilbestrol group that was formerly marketed widely throughout Europe. It is an ester of diethylstilbestrol with propionic acid, and is more slowly absorbed in the body than diethylstilbestrol. The medication has been said to be one of the most potent estrogens known.

Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate, is a natural, endogenous steroid and an estrogen ester. E2S itself is biologically inactive, but it can be converted by steroid sulfatase into estradiol, which is a potent estrogen. Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues. Estrone and E2S are the two immediate metabolic sources of estradiol. E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol. Circulating concentrations of E2S are much lower than those of E1S. High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in feminizing hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.

Prasterone enanthate, also known as dehydroepiandrosterone enanthate (DHEA-E) and sold in combination with estradiol valerate under the brand name Gynodian Depot among others, is a weak androgen, estrogen, and neurosteroid medication which is used as a component of menopausal hormone therapy to treat menopausal symptoms in women. It is available only as an injectable preparation in combination with estradiol valerate. The medication is given by injection into muscle typically once every 4 weeks.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

Polyestriol phosphate, sold under the brand names Gynäsan, Klimadurin, and Triodurin, is an estrogen medication which was previously used in menopausal hormone therapy and is no longer available.

Estriol phosphate (E3P), or estriol 17β-phosphate, also known as estra-1,3,5(10)-triene-3,16α,17β-triol 17β-(dihydrogen phosphate), is an estrogen which was never marketed. It is an estrogen ester, specifically an ester of estriol with phosphoric acid, and acts as a prodrug of estriol by cleavage via phosphatase enzymes in the body. Estriol phosphate is contained within the chemical structure of polyestriol phosphate, and this medication has been marketed for medical use.
















