Estrogen ester Last updated August 11, 2025 Medical uses Estrogen esters are used in hormone therapy , hormonal contraception , and high-dose estrogen therapy (e.g., for prostate cancer and breast cancer ), among other indications. [ 1] [ 2] The first estrogen ester to be marketed was estradiol benzoate in 1933, which was followed by many more. [ 7] [ 8] One of the most widely used estradiol esters is estradiol valerate , which was first introduced in 1954. [ 9] Other major estradiol esters that are or have been used in medicine include estradiol acetate , estradiol cypionate , estradiol dipropionate , estradiol enantate , estradiol undecylate , and polyestradiol phosphate (an estrogen ester polymer), as well as the nitrogen mustard alkylating antineoplastic agent estramustine phosphate (estradiol normustine phosphate). [ 2] [ 10]
The most common vehicles for injections of steroids and steroid esters are oil solutions , but aqueous solutions , aqueous suspensions , and emulsions have also been used. [ 11] [ additional citation(s) needed ] orally , vaginally , or by intravenous injection . [ 11]
Pharmacology Estrogen esters are essentially inactive themselves, with esters such as estradiol valerate and estradiol sulfate having about 2% of the affinity of estradiol for the estrogen receptor . [ 12] Likewise, the estrogen ether mestranol (ethinylestradiol 3-methyl ether) has about 1% of the affinity of estradiol for the estrogen receptor. [ 12] Estrone sulfate has less than 1% of the affinity of estradiol for the estrogen receptor. [ 13] As such, estrogen esters do not bind to the estrogen receptor except at extremely high concentrations. [ 14] The residual affinity of estrogen esters for the estrogen receptor in bioassays may actually be due to conversion into the parent estrogen, as attempts to prevent or limit this conversion have been found to abolish binding to the estrogen receptor and estrogenicity. [ 15] [ 16] [ 17]
Affinities of estrogen receptor ligands for the ERα and ERβ Ligand Other names Relative binding affinities (RBA, %)a Absolute binding affinities (Ki , nM)a Action ERα ERβ ERα ERβ Estradiol E2; 17β-Estradiol 100 100 0.115 (0.04–0.24) 0.15 (0.10–2.08) Estrogen Estrone E1; 17-Ketoestradiol 16.39 (0.7–60) 6.5 (1.36–52) 0.445 (0.3–1.01) 1.75 (0.35–9.24) Estrogen Estriol E3; 16α-OH-17β-E2 12.65 (4.03–56) 26 (14.0–44.6) 0.45 (0.35–1.4) 0.7 (0.63–0.7) Estrogen Estetrol E4; 15α,16α-Di-OH-17β-E2 4.0 3.0 4.9 19 Estrogen Alfatradiol 17α-Estradiol 20.5 (7–80.1) 8.195 (2–42) 0.2–0.52 0.43–1.2 Metabolite 16-Epiestriol 16β-Hydroxy-17β-estradiol 7.795 (4.94–63) 50 ? ? Metabolite 17-Epiestriol 16α-Hydroxy-17α-estradiol 55.45 (29–103) 79–80 ? ? Metabolite 16,17-Epiestriol 16β-Hydroxy-17α-estradiol 1.0 13 ? ? Metabolite 2-Hydroxyestradiol 2-OH-E2 22 (7–81) 11–35 2.5 1.3 Metabolite 2-Methoxyestradiol 2-MeO-E2 0.0027–2.0 1.0 ? ? Metabolite 4-Hydroxyestradiol 4-OH-E2 13 (8–70) 7–56 1.0 1.9 Metabolite 4-Methoxyestradiol 4-MeO-E2 2.0 1.0 ? ? Metabolite 2-Hydroxyestrone 2-OH-E1 2.0–4.0 0.2–0.4 ? ? Metabolite 2-Methoxyestrone 2-MeO-E1 <0.001–<1 <1 ? ? Metabolite 4-Hydroxyestrone 4-OH-E1 1.0–2.0 1.0 ? ? Metabolite 4-Methoxyestrone 4-MeO-E1 <1 <1 ? ? Metabolite 16α-Hydroxyestrone 16α-OH-E1; 17-Ketoestriol 2.0–6.5 35 ? ? Metabolite 2-Hydroxyestriol 2-OH-E3 2.0 1.0 ? ? Metabolite 4-Methoxyestriol 4-MeO-E3 1.0 1.0 ? ? Metabolite Estradiol sulfate E2S; Estradiol 3-sulfate <1 <1 ? ? Metabolite Estradiol disulfate Estradiol 3,17β-disulfate 0.0004 ? ? ? Metabolite Estradiol 3-glucuronide E2-3G 0.0079 ? ? ? Metabolite Estradiol 17β-glucuronide E2-17G 0.0015 ? ? ? Metabolite Estradiol 3-gluc. 17β-sulfate E2-3G-17S 0.0001 ? ? ? Metabolite Estrone sulfate E1S; Estrone 3-sulfate <1 <1 >10 >10 Metabolite Estradiol benzoate EB; Estradiol 3-benzoate 10 ? ? ? Estrogen Estradiol 17β-benzoate E2-17B 11.3 32.6 ? ? Estrogen Estrone methyl ether Estrone 3-methyl ether 0.145 ? ? ? Estrogen ent -Estradiol 1-Estradiol 1.31–12.34 9.44–80.07 ? ? Estrogen Equilin 7-Dehydroestrone 13 (4.0–28.9) 13.0–49 0.79 0.36 Estrogen Equilenin 6,8-Didehydroestrone 2.0–15 7.0–20 0.64 0.62 Estrogen 17β-Dihydroequilin 7-Dehydro-17β-estradiol 7.9–113 7.9–108 0.09 0.17 Estrogen 17α-Dihydroequilin 7-Dehydro-17α-estradiol 18.6 (18–41) 14–32 0.24 0.57 Estrogen 17β-Dihydroequilenin 6,8-Didehydro-17β-estradiol 35–68 90–100 0.15 0.20 Estrogen 17α-Dihydroequilenin 6,8-Didehydro-17α-estradiol 20 49 0.50 0.37 Estrogen Δ8 -Estradiol 8,9-Dehydro-17β-estradiol 68 72 0.15 0.25 Estrogen Δ8 -Estrone 8,9-Dehydroestrone 19 32 0.52 0.57 Estrogen Ethinylestradiol EE; 17α-Ethynyl-17β-E2 120.9 (68.8–480) 44.4 (2.0–144) 0.02–0.05 0.29–0.81 Estrogen Mestranol EE 3-methyl ether ? 2.5 ? ? Estrogen Moxestrol RU-2858; 11β-Methoxy-EE 35–43 5–20 0.5 2.6 Estrogen Methylestradiol 17α-Methyl-17β-estradiol 70 44 ? ? Estrogen Diethylstilbestrol DES; Stilbestrol 129.5 (89.1–468) 219.63 (61.2–295) 0.04 0.05 Estrogen Hexestrol Dihydrodiethylstilbestrol 153.6 (31–302) 60–234 0.06 0.06 Estrogen Dienestrol Dehydrostilbestrol 37 (20.4–223) 56–404 0.05 0.03 Estrogen Benzestrol (B2) – 114 ? ? ? Estrogen Chlorotrianisene TACE 1.74 ? 15.30 ? Estrogen Triphenylethylene TPE 0.074 ? ? ? Estrogen Triphenylbromoethylene TPBE 2.69 ? ? ? Estrogen Tamoxifen ICI-46,474 3 (0.1–47) 3.33 (0.28–6) 3.4–9.69 2.5 SERM Afimoxifene 4-Hydroxytamoxifen; 4-OHT 100.1 (1.7–257) 10 (0.98–339) 2.3 (0.1–3.61) 0.04–4.8 SERM Toremifene 4-Chlorotamoxifen; 4-CT ? ? 7.14–20.3 15.4 SERM Clomifene MRL-41 25 (19.2–37.2) 12 0.9 1.2 SERM Cyclofenil F-6066; Sexovid 151–152 243 ? ? SERM Nafoxidine U-11,000A 30.9–44 16 0.3 0.8 SERM Raloxifene – 41.2 (7.8–69) 5.34 (0.54–16) 0.188–0.52 20.2 SERM Arzoxifene LY-353,381 ? ? 0.179 ? SERM Lasofoxifene CP-336,156 10.2–166 19.0 0.229 ? SERM Ormeloxifene Centchroman ? ? 0.313 ? SERM Levormeloxifene 6720-CDRI; NNC-460,020 1.55 1.88 ? ? SERM Ospemifene Deaminohydroxytoremifene 0.82–2.63 0.59–1.22 ? ? SERM Bazedoxifene – ? ? 0.053 ? SERM Etacstil GW-5638 4.30 11.5 ? ? SERM ICI-164,384 – 63.5 (3.70–97.7) 166 0.2 0.08 Antiestrogen Fulvestrant ICI-182,780 43.5 (9.4–325) 21.65 (2.05–40.5) 0.42 1.3 Antiestrogen Propylpyrazoletriol PPT 49 (10.0–89.1) 0.12 0.40 92.8 ERα agonist 16α-LE2 16α-Lactone-17β-estradiol 14.6–57 0.089 0.27 131 ERα agonist 16α-Iodo-E2 16α-Iodo-17β-estradiol 30.2 2.30 ? ? ERα agonist Methylpiperidinopyrazole MPP 11 0.05 ? ? ERα antagonist Diarylpropionitrile DPN 0.12–0.25 6.6–18 32.4 1.7 ERβ agonist 8β-VE2 8β-Vinyl-17β-estradiol 0.35 22.0–83 12.9 0.50 ERβ agonist Prinaberel ERB-041; WAY-202,041 0.27 67–72 ? ? ERβ agonist ERB-196 WAY-202,196 ? 180 ? ? ERβ agonist Erteberel SERBA-1; LY-500,307 ? ? 2.68 0.19 ERβ agonist SERBA-2 – ? ? 14.5 1.54 ERβ agonist Coumestrol – 9.225 (0.0117–94) 64.125 (0.41–185) 0.14–80.0 0.07–27.0 Xenoestrogen Genistein – 0.445 (0.0012–16) 33.42 (0.86–87) 2.6–126 0.3–12.8 Xenoestrogen Equol – 0.2–0.287 0.85 (0.10–2.85) ? ? Xenoestrogen Daidzein – 0.07 (0.0018–9.3) 0.7865 (0.04–17.1) 2.0 85.3 Xenoestrogen Biochanin A – 0.04 (0.022–0.15) 0.6225 (0.010–1.2) 174 8.9 Xenoestrogen Kaempferol – 0.07 (0.029–0.10) 2.2 (0.002–3.00) ? ? Xenoestrogen Naringenin – 0.0054 (<0.001–0.01) 0.15 (0.11–0.33) ? ? Xenoestrogen 8-Prenylnaringenin 8-PN 4.4 ? ? ? Xenoestrogen Quercetin – <0.001–0.01 0.002–0.040 ? ? Xenoestrogen Ipriflavone – <0.01 <0.01 ? ? Xenoestrogen Miroestrol – 0.39 ? ? ? Xenoestrogen Deoxymiroestrol – 2.0 ? ? ? Xenoestrogen β-Sitosterol – <0.001–0.0875 <0.001–0.016 ? ? Xenoestrogen Resveratrol – <0.001–0.0032 ? ? ? Xenoestrogen α-Zearalenol – 48 (13–52.5) ? ? ? Xenoestrogen β-Zearalenol – 0.6 (0.032–13) ? ? ? Xenoestrogen Zeranol α-Zearalanol 48–111 ? ? ? Xenoestrogen Taleranol β-Zearalanol 16 (13–17.8) 14 0.8 0.9 Xenoestrogen Zearalenone ZEN 7.68 (2.04–28) 9.45 (2.43–31.5) ? ? Xenoestrogen Zearalanone ZAN 0.51 ? ? ? Xenoestrogen Bisphenol A BPA 0.0315 (0.008–1.0) 0.135 (0.002–4.23) 195 35 Xenoestrogen Endosulfan EDS <0.001–<0.01 <0.01 ? ? Xenoestrogen Kepone Chlordecone 0.0069–0.2 ? ? ? Xenoestrogen o,p' -DDT – 0.0073–0.4 ? ? ? Xenoestrogen p,p' -DDT – 0.03 ? ? ? Xenoestrogen Methoxychlor p,p' -Dimethoxy-DDT0.01 (<0.001–0.02) 0.01–0.13 ? ? Xenoestrogen HPTE Hydroxychlor; p,p' -OH-DDT 1.2–1.7 ? ? ? Xenoestrogen Testosterone T; 4-Androstenolone <0.0001–<0.01 <0.002–0.040 >5000 >5000 Androgen Dihydrotestosterone DHT; 5α-Androstanolone 0.01 (<0.001–0.05) 0.0059–0.17 221–>5000 73–1688 Androgen Nandrolone 19-Nortestosterone; 19-NT 0.01 0.23 765 53 Androgen Dehydroepiandrosterone DHEA; Prasterone 0.038 (<0.001–0.04) 0.019–0.07 245–1053 163–515 Androgen 5-Androstenediol A5; Androstenediol 6 17 3.6 0.9 Androgen 4-Androstenediol – 0.5 0.6 23 19 Androgen 4-Androstenedione A4; Androstenedione <0.01 <0.01 >10000 >10000 Androgen 3α-Androstanediol 3α-Adiol 0.07 0.3 260 48 Androgen 3β-Androstanediol 3β-Adiol 3 7 6 2 Androgen Androstanedione 5α-Androstanedione <0.01 <0.01 >10000 >10000 Androgen Etiocholanedione 5β-Androstanedione <0.01 <0.01 >10000 >10000 Androgen Methyltestosterone 17α-Methyltestosterone <0.0001 ? ? ? Androgen Ethinyl-3α-androstanediol 17α-Ethynyl-3α-adiol 4.0 <0.07 ? ? Estrogen Ethinyl-3β-androstanediol 17α-Ethynyl-3β-adiol 50 5.6 ? ? Estrogen Progesterone P4; 4-Pregnenedione <0.001–0.6 <0.001–0.010 ? ? Progestogen Norethisterone NET; 17α-Ethynyl-19-NT 0.085 (0.0015–<0.1) 0.1 (0.01–0.3) 152 1084 Progestogen Norethynodrel 5(10)-Norethisterone 0.5 (0.3–0.7) <0.1–0.22 14 53 Progestogen Tibolone 7α-Methylnorethynodrel 0.5 (0.45–2.0) 0.2–0.076 ? ? Progestogen Δ4 -Tibolone 7α-Methylnorethisterone 0.069–<0.1 0.027–<0.1 ? ? Progestogen 3α-Hydroxytibolone – 2.5 (1.06–5.0) 0.6–0.8 ? ? Progestogen 3β-Hydroxytibolone – 1.6 (0.75–1.9) 0.070–0.1 ? ? Progestogen Footnotes: a = (1) Binding affinity values are of the format "median (range)" (# (#–#)), "range" (#–#), or "value" (#) depending on the values available. The full sets of values within the ranges can be found in the Wiki code. (2) Binding affinities were determined via displacement studies in a variety of in-vitro systems with labeled estradiol and human ERα and ERβ proteins (except the ERβ values from Kuiper et al. (1997), which are rat ERβ). Sources: See template page.
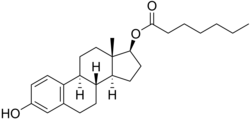
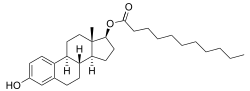
In general, the longer the fatty acid ester chain of an estrogen ester, the greater its lipophilicity , and the longer the duration of the estrogen ester with intramuscular injection. [ 1] [ 10] It has been said that, via intramuscular injection, the duration of estradiol benzoate (with an ester of length 1 carbon plus a benzene ring ) is 2 to 3 days, of estradiol dipropionate (with two esters each of length 2 carbons) is 1 to 2 weeks, of estradiol valerate (ester of 5 carbons) is 1 to 3 weeks, and of estradiol cypionate (ester of 3 carbons plus a cyclopentane ring) is 3 to 4 weeks. [ 18] Estradiol enantate (ester of 7 carbons) has a duration of around 20 days. [ 2] [ 19] [ 20] Likewise, estradiol undecylate (ester of 10 carbons) has a very extended duration, which is longer than that of all of the aforementioned esters. [ 10] [ 21] [ 22]
Polyestradiol phosphate is an atypical estradiol ester. [ 23] [ 24] It is a phosphoric acid ester of estradiol in the form of a polymer , with an average polymer chain length of approximately 13 repeat units of estradiol phosphate . [ 23] It is slowly cleaved into estradiol and phosphoric acid by phosphatases . [ 23] Compared to conventional estradiol esters, polyestradiol phosphate has an extremely long duration; its elimination half-life is approximately 70 days. [ 24] Whereas conventional estradiol esters form a long-lasting depot in muscle and fat at the site of injection, [ 1] this is not the case with polyestradiol phosphate. [ 25] Instead, polyestradiol phosphate is taken up rapidly into the bloodstream following injection (by 90% within 24 hours), where it circulates, and is accumulated in the reticuloendothelial system . [ 25] Unlike other estradiol esters, polyestradiol phosphate is resistant to hydrolysis, which may be because it is a phosphatase inhibitor and may inhibit its own metabolism . [ 23]
Estrogen esters also occur naturally in the body, for instance estrogen conjugates like estrone sulfate and estrone glucuronide and the very long-lived lipoidal estradiol , which is constituted by ultra-long-chain esters like estradiol palmitate (ester of 16 carbons) and estradiol stearate (ester of 18 carbons). [ 1] [ 2] [ 26]
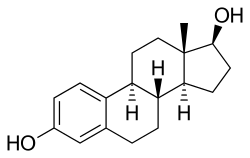
Chemistry Estradiol plus the fatty acid valeric acid (valerate) equals estradiol valerate , a C17β ester of estradiol and one of the most widely used estrogen esters. Polyestradiol phosphate , a polymer of estradiol phosphate , the C17β phosphoric acid ester of estradiol . It has on average of 13 repeat units . Estradiol esters have an ester moiety , usually a straight-chain fatty acid (e.g., valeric acid ) or an aromatic fatty acid (e.g., benzoic acid ), attached at the C3 and/or C17β positions of the steroid nucleus . These alkoxy moieties are substituted in place of the hydroxyl groups present in the unesterified estradiol molecule. Fatty acid esters serve to increase the lipophilicity of estradiol, increasing its solubility in fat . This causes them to form a depot with intramuscular or subcutaneous injection and gives them a long duration when administered by these routes.
Some estradiol esters have other moieties instead of fatty acids as the esters. Such esters include sulfuric acid (as in estradiol sulfate ), sulfamic acid (as in estradiol sulfamate ), phosphoric acid (as in estradiol phosphate ), glucuronic acid (as in estradiol glucuronide , and others (e.g., estramustine phosphate (estradiol 3-normustine 17β-phosphate)). These esters are all hydrophilic , and have greater water solubility than estradiol or fatty acid estradiol esters. Unlike fatty acid estradiol esters, water-soluble estradiol esters can be administered by intravenous injection .
A few estrogen esters are polymers . These include polyestradiol phosphate and polyestriol phosphate , which are polymers of estradiol phosphate and estriol phosphate monomers , respectively. The monomers are connected in both cases by phosphate groups via the C3 and C17β positions. Polyestradiol phosphate has an average polymer chain length of approximately 13 repeat units of estradiol phosphate. [ 23] That is, each polyestradiol phosphate molecule is a polymer consisting on average of 13 estradiol phosphate molecules bonded together. [ 23] These polymeric estrogen esters are hydrophilic and water-soluble. Upon intramuscular injection, they do not form a depot and instead are rapidly absorbed into the circulation. However, they are only slowly cleaved into monomers, and as a result, have a very long duration in the body even outlasting that of many longer-chain fatty-acid estrogen esters.
Chemical structures of estradiol and major estradiol esters
Structural properties of selected estradiol esters Estrogen Structure Ester(s) Relativemol. weight RelativeE2 contentb log Pc Position(s) Moiet(ies) Type Lengtha Estradiol – – – – 1.00 1.00 4.0 Estradiol acetate C3 Ethanoic acid Straight-chain fatty acid 2 1.15 0.87 4.2 Estradiol benzoate C3 Benzoic acid Aromatic fatty acid – (~4–5) 1.38 0.72 4.7 Estradiol dipropionate C3, C17β Propanoic acid (×2) Straight-chain fatty acid 3 (×2) 1.41 0.71 4.9 Estradiol valerate C17β Pentanoic acid Straight-chain fatty acid 5 1.31 0.76 5.6–6.3 Estradiol benzoate butyrate C3, C17β Benzoic acid , butyric acid Mixed fatty acid – (~6, 2) 1.64 0.61 6.3 Estradiol cypionate C17β Cyclopentylpropanoic acid Cyclic fatty acid – (~6) 1.46 0.69 6.9 Estradiol enanthate C17β Heptanoic acid Straight-chain fatty acid 7 1.41 0.71 6.7–7.3 Estradiol dienanthate C3, C17β Heptanoic acid (×2) Straight-chain fatty acid 7 (×2) 1.82 0.55 8.1–10.4 Estradiol undecylate C17β Undecanoic acid Straight-chain fatty acid 11 1.62 0.62 9.2–9.8 Estradiol stearate C17β Octadecanoic acid Straight-chain fatty acid 18 1.98 0.51 12.2–12.4 Estradiol distearate C3, C17β Octadecanoic acid (×2) Straight-chain fatty acid 18 (×2) 2.96 0.34 20.2 Estradiol sulfate C3 Sulfuric acid Water-soluble conjugate – 1.29 0.77 0.3–3.8 Estradiol glucuronide C17β Glucuronic acid Water-soluble conjugate – 1.65 0.61 2.1–2.7 Estramustine phosphate d C3, C17β Normustine , phosphoric acid Water-soluble conjugate – 1.91 0.52 2.9–5.0 Polyestradiol phosphate e C3–C17β Phosphoric acid Water-soluble conjugate – 1.23f 0.81f 2.9g Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity /hydrophobicity ). Retrieved from PubChem , ChemSpider , and DrugBank . d = Also known as estradiol normustine phosphate . e = Polymer of estradiol phosphate (~13 repeat units ). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles.
References 1 2 3 4 5 6 7 8 9 10 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF) . Climacteric . 8 (Suppl 1): 3– 63. doi :10.1080/13697130500148875 . PMID 16112947 . S2CID 24616324 . 1 2 3 4 5 6 7 8 9 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen 235– 237, 261, 271. ISBN 978-3-642-60107-1 Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens. 1 2 R. S. Satoskar; S. D. Bhandarkar &nirmala N. Rege (1969). Pharmacology And Pharmacotherapeutics (New Revised 21 St Ed.) 24. ISBN 978-81-7991-527-1 . Retrieved 29 May 2012 . ↑ Gordon L. Amidon; Ping I. Lee; Elizabeth M. Topp (2000). Transport Processes in Pharmaceutical Systems 188– 189. ISBN 978-0-8247-6610-8 . Retrieved 29 May 2012 . ↑ Parkes AS (February 1938). "Effective Absorption of Hormones" . Br Med J . 1 (4024): 371– 3. doi :10.1136/bmj.1.4024.371 . PMC 2085798 . PMID 20781252 . ↑ Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas . 4 (4): 315– 24. doi :10.1016/0378-5122(82)90064-0 . PMID 7169965 . ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies 897–. ISBN 978-1-4757-2085-3 ↑ Index Nominum 2000: International Drug Directory 404– 406. ISBN 978-3-88763-075-1 . Retrieved 13 September 2012 . ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition 1477–. ISBN 978-0-8155-1856-3 1 2 3 Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception . 21 (4): 415– 24. doi :10.1016/s0010-7824(80)80018-7 . PMID 7389356 . 1 2 C. W. Emmens (22 October 2013). Hormone Assay 394– 395. ISBN 978-1-4832-7286-3 1 2 Gudermann, T. (2005). "Endokrinpharmakologie". Klinische Endokrinologie für Frauenärzte . pp. 187– 220. doi :10.1007/3-540-26406-X_10 . ISBN 3-540-44162-X ↑ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (March 1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta" . Endocrinology . 138 (3): 863– 70. doi : 10.1210/endo.138.3.4979 . PMID 9048584 . ↑ Hochberg RB (June 1998). "Biological esterification of steroids" . Endocr. Rev . 19 (3): 331– 48. doi : 10.1210/edrv.19.3.0330 . PMID 9626557 . ↑ Janocko L, Larner JM, Hochberg RB (April 1984). "The interaction of C-17 esters of estradiol with the estrogen receptor". Endocrinology . 114 (4): 1180– 6. doi :10.1210/endo-114-4-1180 . PMID 6705734 . ↑ Bjerregaard-Olesen C, Ghisari M, Kjeldsen LS, Wielsøe M, Bonefeld-Jørgensen EC (January 2016). "Estrone sulfate and dehydroepiandrosterone sulfate: Transactivation of the estrogen and androgen receptor". Steroids . 105 : 50– 8. doi :10.1016/j.steroids.2015.11.009 . PMID 26666359 . S2CID 46663814 . ↑ Clark, Barbara J.; Prough, Russell A.; Klinge, Carolyn M. (2018). "Mechanisms of Action of Dehydroepiandrosterone". Dehydroepiandrosterone . Vitamins and Hormones. Vol. 108. pp. 29– 73. doi :10.1016/bs.vh.2018.02.003 . ISBN 9780128143612 ISSN 0083-6729 . PMID 30029731 . ↑ H.J. Buchsbaum (6 December 2012). The Menopause 62–. ISBN 978-1-4612-5525-3 ↑ Recio R, Garza-Flores J, Schiavon R, Reyes A, Diaz-Sanchez V, Valles V, Luz de la Cruz D, Oropeza G, Perez-Palacios G (June 1986). "Pharmacodynamic assessment of dihydroxyprogesterone acetophenide plus estradiol enanthate as a monthly injectable contraceptive". Contraception . 33 (6): 579– 89. doi :10.1016/0010-7824(86)90046-6 . PMID 3769482 . ↑ Wiemeyer JC, Fernandez M, Moguilevsky JA, Sagasta CL (1986). "Pharmacokinetic studies of estradiol enantate in menopausic women". Arzneimittelforschung . 36 (11): 1674– 7. PMID 3814225 . ↑ Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg . 30 (1): 48– 55. doi :10.1080/17843286.1975.11716973 . PMID 1231448 . ↑ R. S. Satoskar; S. D. Bhandarkar &nirmala N. Rege (1973). Pharmacology and Pharmacotherapeutics 934–. ISBN 978-81-7991-527-1 1 2 3 4 5 6 1 2 1 2 Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). Vol. 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-98-46-3 ↑ Hochberg RB, Pahuja SL, Larner JM, Zielinski JE (1990). "Estradiol-fatty acid esters. Endogenous long-lived estrogens". Ann. N. Y. Acad. Sci . 595 (1): 74– 92. Bibcode :1990NYASA.595...74H . doi :10.1111/j.1749-6632.1990.tb34284.x . PMID 2197972 . S2CID 19866729 . ↑ Shellenberger, T. E. (1986). "Pharmacology of estrogens". The Climacteric in Perspective . pp. 393– 410. doi :10.1007/978-94-009-4145-8_36 . ISBN 978-94-010-8339-3 Further reading
Estrogens
ER Tooltip Estrogen receptor agonists Steroidal: Alfatradiol Certain androgens /anabolic steroids (e.g., testosterone , testosterone esters , methyltestosterone , metandienone , nandrolone esters ) (via estrogenic metabolites) Certain progestins (e.g., norethisterone , noretynodrel , etynodiol diacetate , tibolone ) Clomestrone Cloxestradiol acetate Conjugated estriol Conjugated estrogens Epiestriol Epimestrol Esterified estrogens Estetrol † Estradiol Estradiol esters (e.g., estradiol acetate , estradiol benzoate , estradiol cypionate , estradiol enanthate , estradiol undecylate , estradiol valerate , polyestradiol phosphate , estradiol ester mixtures (Climacteron )) Estramustine phosphate Estriol Estriol esters (e.g., estriol succinate , polyestriol phosphate ) Estrogenic substances Estrone Estrone esters Ethinylestradiol # Hydroxyestrone diacetate Mestranol Methylestradiol Moxestrol Nilestriol Prasterone (dehydroepiandrosterone; DHEA) Promestriene Quinestradol Quinestrol Progonadotropins
Antiestrogens
ER Tooltip Estrogen receptor antagonistsSERMs Tooltip selective estrogen receptor modulators /SERDs Tooltip selective estrogen receptor downregulators ) Aromatase inhibitors Antigonadotropins Androgens /anabolic steroids (e.g., testosterone , testosterone esters , nandrolone esters , oxandrolone , fluoxymesterone ) D2 receptor antagonists (prolactin releasers) (e.g., domperidone , metoclopramide , risperidone , haloperidol , chlorpromazine , sulpiride ) GnRH agonistsleuprorelin , goserelin ) GnRH antagonistscetrorelix , elagolix ) Progestogens (e.g., chlormadinone acetate , cyproterone acetate , gestonorone caproate , hydroxyprogesterone caproate , medroxyprogesterone acetate , megestrol acetate ) Others
ER Tooltip Estrogen receptor
Agonists Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol ) 17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites) Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -Estradiol Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol Estrofurate Estrogenic substances Estromustine Estrone Etamestrol (eptamestrol) Ethinylandrostenediol Ethinylestradiol Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole ) Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone ) Coumestans (e.g., coumestrol , psoralidin ) Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin ) Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) ) Metalloestrogens (e.g., cadmium ) Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan ) Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone ) Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol ) Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) ) Steroid -like (e.g., deoxymiroestrol , miroestrol ) Stilbenoids (e.g., resveratrol , rhaponticin ) Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs ) Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.