Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonists/antagonists (ERAAs), are a class of drugs that act on estrogen receptors (ERs). Compared to pure ER agonists–antagonists, SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen-like action in various tissues.

Estrogen receptors (ERs) are proteins found in cells that function as receptors for the hormone estrogen (17β-estradiol). There are two main classes of ERs. The first includes the intracellular estrogen receptors, namely ERα and ERβ, which belong to the nuclear receptor family. The second class consists of membrane estrogen receptors (mERs), such as GPER (GPR30), ER-X, and Gq-mER, which are primarily G protein-coupled receptors. This article focuses on the nuclear estrogen receptors.
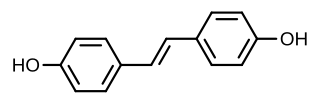
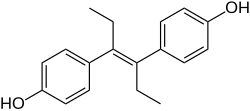
Stilbestrol, or stilboestrol, also known as 4,4'-dihydroxystilbene or 4,4'-stilbenediol, is a stilbenoid nonsteroidal estrogen and the parent compound of a group of more potent nonsteroidal estrogen derivatives that includes, most notably, diethylstilbestrol (DES). The term "stilbestrol" is often used incorrectly to refer to DES, but they are not the same compound.
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.

Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production. Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development.
Mycoestrogens are xenoestrogens produced by fungi. They are sometimes referred to as mycotoxins. Among important mycoestrogens are zearalenone, zearalenol and zearalanol. Although all of these can be produced by various Fusarium species, zearalenol and zearalanol may also be produced endogenously in ruminants that have ingested zearalenone. Alpha-zearalanol is also produced semisynthetically, for veterinary use; such use is prohibited in the European Union.

Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT) and by its tentative brand name TamoGel, is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and an active metabolite of tamoxifen. The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast. It has completed a phase II clinical trial for cyclical mastalgia, but further studies are required before afimoxifene can be approved for this indication and marketed.

Neurodegenerative diseases can disrupt the normal human homeostasis and result in abnormal estrogen levels. For example, neurodegenerative diseases can cause different physiological effects in males and females. In particular, estrogen studies have revealed complex interactions with neurodegenerative diseases. Estrogen was initially proposed to be a possible treatment for certain types of neurodegenerative diseases but a plethora of harmful side effects such as increased susceptibility to breast cancer and coronary heart disease overshadowed any beneficial outcomes. On the other hand, Estrogen Replacement Therapy has shown some positive effects with postmenopausal women. Estrogen and estrogen-like molecules form a large family of potentially beneficial alternatives that can have dramatic effects on human homeostasis and disease. Subsequently, large-scale efforts were initiated to screen for useful estrogen family molecules. Furthermore, scientists discovered new ways to synthesize estrogen-like compounds that can avoid many side effects. These are called nonsteroidal estrogens, which come from natural or synthetic products including plants, fungi, and chemicals.
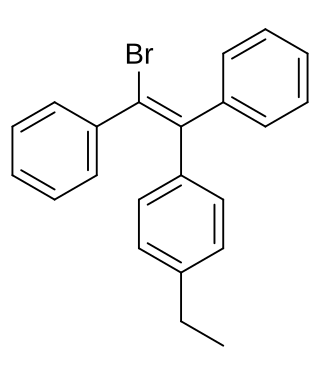
Broparestrol, also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active, and are similarly antiestrogenic but, unlike broparestrol, were never marketed.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Estrobin, also known as α,α-di(p-ethoxyphenyl)-β-phenylbromoethylene and commonly abbreviated as DBE, is a synthetic, nonsteroidal estrogen of the triphenylethylene group that was never marketed. Chlorotrianisene, and subsequently clomifene and tamoxifen, were derived from it. Estrobin, similarly to other triphenylethylenes, is very lipophilic and hence very long-lasting in its duration of action. Similarly to chlorotrianisene, estrobin behaves a prodrug to a much more potent estrogen in the body.

Ethamoxytriphetol is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen to be discovered. It has been described as "essentially devoid of estrogenic activity" and as having "very low estrogenic activity in all species tested". However, some estrogenic effects in the uterus have been observed, so it is not a pure antiestrogen but is, instead, technically a selective estrogen receptor modulator (SERM). For all intents and purposes, it is a nearly pure antiestrogen, however.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.
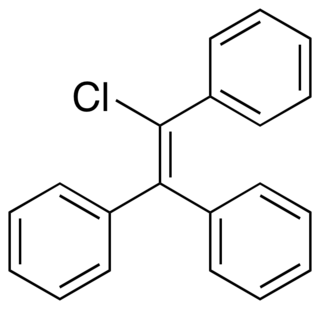
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.
SERBA-2, short for selective estrogen receptor beta agonist-2, is a synthetic, nonsteroidal estrogen which acts as a selective ERβ agonist. For the ERα and ERβ, SERBA-2 has affinities (Ki) of 14.5 nM and 1.54 nM, efficacies of 85% and 100%, and EC50 values of 85 nM and 3.61 nM, respectively, demonstrating 9-fold binding selectivity and 11-fold functional selectivity for the ERβ over the ERα. An enantiomer of SERBA-2, erteberel (SERBA-1), is more potent and selective in comparison and is under development for the treatment of schizophrenia.

(S,S)-Tetrahydrochrysene ((S,S)-THC) is a steroid-like nonsteroidal estrogen and agonist of both the estrogen receptors, ERα and ERβ. It is an enantiomer of (R,R)-tetrahydrochrysene ((R,R)-THC), which, in contrast, is an ERβ silent antagonist and ERα agonist with 10-fold selectivity (i.e., affinity) for the ERβ over the ERα and with 20-fold greater affinity for the ERβ relative to that of (S,S)-THC.

Nitromifene (INNTooltip International Nonproprietary Name; also as the citrate salt nitromifene citrate (USANTooltip United States Adopted Name), developmental code names CI-628, CN-5518, CN-55945) is a nonsteroidal selective estrogen receptor modulator (SERM) related to triphenylethylenes like tamoxifen that was never marketed. It is a mixture of (E)- and (Z)-isomers that possess similar antiestrogenic activity. The drug was described in 1966. Along with tamoxifen, nafoxidine, and clomifene, it was one of the earliest SERMs.

4'-Hydroxynorendoxifen is a synthetic, nonsteroidal antiestrogen of the triphenylethylene group. It is a dual selective estrogen receptor modulator (SERM) and aromatase inhibitor (AI), and was derived from tamoxifen, a SERM, and norendoxifen, a metabolite of tamoxifen that has been found to act as an AI. The drug has been suggested for potential development as a treatment for estrogen receptor (ER)-positive breast cancer. It was synthesized in 2015.