Contents
- Biosynthesis
- Stimulation
- Biological function
- Mineralocorticoid receptors
- Control of aldosterone release from the adrenal cortex
- Major regulators
- Miscellaneous regulators
- Aldosterone feedback
- Associated clinical conditions
- Hyperaldosteronism
- Hypoaldosteronism
- Additional images
- References
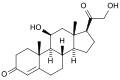
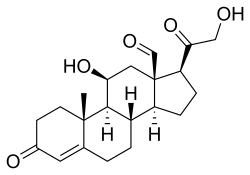 Skeletal formula of the fictitious aldehyde form (exists as tautomeric mixture in solvent) [1] | |
 | |
| Names | |
|---|---|
| IUPAC name 11β,21-Dihydroxy-3,20-dioxopregn-4-en-18-al | |
| Systematic IUPAC name (1S,3aS,3bS,9aR,9bS,10S,11aR)-10-Hydroxy-1-(hydroxyacetyl)-9a-methyl-7-oxo-1,2,3,3a,3b,4,5,7,8,9,9a,9b,10,11-tetradecahydro-11aH-cyclopenta[a]phenanthrene-11a-carbaldehyde | |
| Other names Aldocorten; Aldocortin; Electrocortin; Reichstein X; 18-Aldocorticosterone; 18-Oxocorticosterone | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.128 |
| KEGG | |
| MeSH | Aldosterone |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C21H28O5 | |
| Molar mass | 360.450 g·mol−1 |
| Pharmacology | |
| H02AA01 ( WHO ) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. [4] [5] It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. [6] It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. [6] It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. [7] When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. [8] Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart. [7]
Aldosterone is part of the renin–angiotensin–aldosterone system. It has a plasma half-life of less than 20 minutes. [9] Drugs that interfere with the secretion or action of aldosterone are in use as antihypertensives, like lisinopril, which lowers blood pressure by blocking the angiotensin-converting enzyme (ACE), leading to lower aldosterone secretion. The net effect of these drugs is to reduce sodium and water retention but increase the retention of potassium. In other words, these drugs stimulate the excretion of sodium and water in urine, while they block the excretion of potassium.
Another example is spironolactone, a potassium-sparing diuretic of the steroidal spirolactone group, which interferes with the aldosterone receptor (among others) leading to lower blood pressure by the mechanism described above.
Aldosterone was first isolated by Sylvia Tait (Simpson) and Jim Tait in 1953; in collaboration with Tadeusz Reichstein. [10] [11] [12]