An arterial blood gas (ABG) test, or arterial blood gas analysis (ABGA) measures the amounts of arterial gases, such as oxygen and carbon dioxide. An ABG test requires that a small volume of blood be drawn from the radial artery with a syringe and a thin needle, but sometimes the femoral artery in the groin or another site is used. The blood can also be drawn from an arterial catheter.
Acidosis is a biological process producing hydrogen ions and increasing their concentration in blood or body fluids. pH is the negative log of hydrogen ion concentration and so it is decreased by a process of acidosis.
Alkalosis is the result of a process reducing hydrogen ion concentration of arterial blood plasma (alkalemia). In contrast to acidemia, alkalemia occurs when the serum pH is higher than normal. Alkalosis is usually divided into the categories of respiratory alkalosis and metabolic alkalosis or a combined respiratory/metabolic alkalosis.

Hypercapnia (from the Greek hyper, "above" or "too much" and kapnos, "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous product of the body's metabolism and is normally expelled through the lungs. Carbon dioxide may accumulate in any condition that causes hypoventilation, a reduction of alveolar ventilation (the clearance of air from the small sacs of the lung where gas exchange takes place) as well as resulting from inhalation of CO2. Inability of the lungs to clear carbon dioxide, or inhalation of elevated levels of CO2, leads to respiratory acidosis. Eventually the body compensates for the raised acidity by retaining alkali in the kidneys, a process known as "metabolic compensation".
The control of ventilation is the physiological mechanisms involved in the control of breathing, which is the movement of air into and out of the lungs. Ventilation facilitates respiration. Respiration refers to the utilization of oxygen and balancing of carbon dioxide by the body as a whole, or by individual cells in cellular respiration.

The carotid body is a small cluster of peripheral chemoreceptor cells and supporting sustentacular cells situated at the bifurcation of each common carotid artery in its tunica externa.
Hypocapnia, also known as hypocarbia, sometimes incorrectly called acapnia, is a state of reduced carbon dioxide in the blood. Hypocapnia usually results from deep or rapid breathing, known as hyperventilation.

Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.

Respiratory acidosis is a state in which decreased ventilation (hypoventilation) increases the concentration of carbon dioxide in the blood and decreases the blood's pH.

Respiratory alkalosis is a medical condition in which increased respiration elevates the blood pH beyond the normal range (7.35–7.45) with a concurrent reduction in arterial levels of carbon dioxide. This condition is one of the four primary disturbances of acid–base homeostasis.

Metabolic alkalosis is an acid-base disorder in which the pH of tissue is elevated beyond the normal range (7.35–7.45). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. The condition typically cannot last long if the kidneys are functioning properly.
In physiology, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base present in the blood. The value is usually reported as a concentration in units of mEq/L (mmol/L), with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2 mEq/L.
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases in the ECF is crucial for the normal physiology of the body—and for cellular metabolism. The pH of the intracellular fluid and the extracellular fluid need to be maintained at a constant level.
The factors that determine the values for alveolar pO2 and pCO2 are:
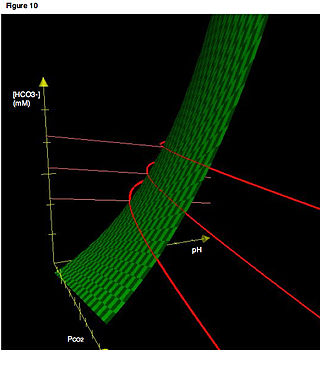
In acid base physiology, the Davenport diagram is a graphical tool, developed by Horace W. Davenport, that allows a clinician or investigator to describe blood bicarbonate concentrations and blood pH following a respiratory and/or metabolic acid-base disturbance. The diagram depicts a three-dimensional surface describing all possible states of chemical equilibria between gaseous carbon dioxide, aqueous bicarbonate and aqueous protons at the physiologically complex interface of the alveoli of the lungs and the alveolar capillaries. Although the surface represented in the diagram is experimentally determined, the Davenport diagram is rarely used in the clinical setting, but allows the investigator to envision the effects of physiological changes on blood acid-base chemistry. For clinical use there are two recent innovations: an Acid-Base Diagram which provides Text Descriptions for the abnormalities and a High Altitude Version that provides text descriptions appropriate for the altitude.
Normal anion gap acidosis is an acidosis that is not accompanied by an abnormally increased anion gap.

pCO2, pCO2, or is the partial pressure of carbon dioxide (CO2), often used in reference to blood but also used in meteorology, climate science, oceanography, and limnology to describe the fractional pressure of CO2 as a function of its concentration in gas or dissolved phases. The units of pCO2 are mmHg, atm, torr, Pa, or any other standard unit of atmospheric pressure. The pCO2 of Earth's atmosphere has risen from approximately 280 ppm (parts-per-million) to a mean 2019 value of 409.8 ppm as a result of anthropogenic release of carbon dioxide from fossil fuel burning. This is the highest atmospheric concentration to have existed on Earth for at least the last 800,000 years.

Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range. In the fetus, the normal range differs based on which umbilical vessel is sampled. It can exist in varying levels of severity, some life-threatening.
Winters's formula, named after R. W. Winters, is a formula used to evaluate respiratory compensation when analyzing acid-base disorders in the presence of metabolic acidosis. It can be given as:

Salicylate poisoning, also known as aspirin poisoning, is the acute or chronic poisoning with a salicylate such as aspirin. The classic symptoms are ringing in the ears, nausea, abdominal pain, and a fast breathing rate. Early on, these may be subtle, while larger doses may result in fever. Complications can include swelling of the brain or lungs, seizures, low blood sugar, or cardiac arrest.








