| |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌveɪzoʊˈprɛsɪn/ |
| Other names | Antidiuretic hormone (ADH); arginine vasopressin (AVP); argipressin |
| ATC code | |
| Physiological data | |
| Source tissues | Supraoptic nucleus; paraventricular nucleus of hypothalamus |
| Target tissues | System-wide |
| Receptors | V1A, V1B, V2, OXTR |
| Agonists | Felypressin, desmopressin |
| Antagonists | Diuretics |
| Metabolism | Predominantly in the liver and kidneys |
| Pharmacokinetic data | |
| Protein binding | 1% |
| Metabolism | Predominantly in the liver and kidneys |
| Elimination half-life | 10–20 minutes |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
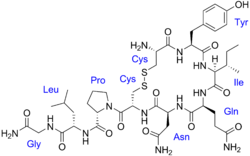
| Formula | C46H65N15O12S2 |
| Molar mass | 1084.24 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.6±0.1 g/cm3 |
| |
| |
Mammalian vasopressin, also called antidiuretic hormone (ADH) [5] , arginine vasopressin (AVP) or argipressin, [6] is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, [7] and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure. [8] [9] [10]
Contents
- Physiology
- Function
- Regulation
- Production and secretion
- Vasopressin during surgery and anaesthesia
- Receptors
- Structure and relation to oxytocin
- Medical use
- Pharmacokinetics
- Side effects
- Contraindications
- Interactions
- Deficiency
- Excess
- History
- Animal studies
- Human studies
- See also
- References
- Further reading
A third function is possible. Some AVP may be released directly into the brain from the hypothalamus, and may play an important role in social behavior, sexual motivation and pair bonding, and maternal responses to stress. [11]
Vasopressin induces differentiation of stem cells into cardiomyocytes and promotes heart muscle homeostasis. [12]
It has a very short half-life, between 16 and 24 minutes. [10]