Function
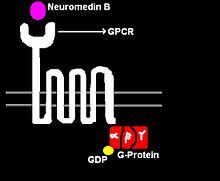

Neuromedin regulates the following functions:
| neuromedin B | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | NMB | ||||||
| NCBI gene | 4828 | ||||||
| HGNC | 7842 | ||||||
| OMIM | 162340 | ||||||
| RefSeq | NM_021077 | ||||||
| UniProt | P08949 | ||||||
| Other data | |||||||
| Locus | Chr. 15 q11-qter | ||||||
| |||||||
Neuromedin B (NMB) is a bombesin-related peptide in mammals. [1] [2] It was originally purified from pig spinal cord, and later shown to be present in human central nervous system and gastrointestinal tract. [3]
The sequence of the C-terminal decapeptide is highly conserved across mammalian species: GNLWATGHFM-(NH2); this decapeptide is sometimes noted as neuromedin B, but it is more accurately described as neuromedin B 23-32. The sequence of neuromedin B (in rat) is: TPFSWDLPEPRSRASKIRVHPRGNLWATGHFM-(NH2). [4] The (NH2) here indicates a post-translational modification -- alpha amidation of the carboxy terminus.


Neuromedin regulates the following functions:
NMB acts by binding to its high affinity cell surface receptor, neuromedin B receptor (NMBR). This receptor is a G protein-coupled receptor with seven transmembrane spanning regions, hence the receptor is also denoted as a 7-transmembrane receptor (7-TMR). Upon binding several intracellular signaling pathways are triggered (see Figure 2).
When NMB binds to its 7-TMR, the heterotrimeric G protein that is attached to the receptor is activated. The G-protein is called heterotrimeric because it consists of 3 polypeptides: α subunit, β subunit, and γ subunit. In the activated NMBR/G-protein complex, there occurs an exchange of GTP for GDP bound to G-α subunit. The G-α subunit, in turn, dissociated form the G-βγ subunits. The free G-α inactivates adenylate cyclase (AC), which, in turn, catalyzes the conversion of ATP to cAMP, the latter of which functioning as a second messenger. cAMP activates of the enzyme Protein Kinase A (PKA). PKA enters the nucleus and activates the cAMP response element-binding protein. The activated CREB binds along with CREB binding protein, co-activator to the CRE region of the DNA in the nucleus. CREB and CBP are held together by leucine zippers. CRE is the control that activates number of growth factors, and thus cell proliferation and some anti-apoptotic genes. In the brain, CREB plays a role in long-term memory and learning.

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.

Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.

In cell biology, protein kinase A (PKA) is a family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase.
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.
Bombesin is a 14-amino acid peptide originally isolated from the skin of the European fire-bellied toad by Vittorio Erspamer et al. and named after its source. It has two known homologs in mammals called neuromedin B and gastrin-releasing peptide. It stimulates gastrin release from G cells. It activates three different G-protein-coupled receptors known as BBR1, -2, and -3. It also activates these receptors in the brain. Together with cholecystokinin, it is the second major source of negative feedback signals that stop eating behaviour.

Gastrin-releasing peptide, also known as GRP, is a neuropeptide, a regulatory molecule that has been implicated in a number of physiological and pathophysiological processes. Most notably, GRP stimulates the release of gastrin from the G cells of the stomach.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.
Growth hormone–releasing hormone (GHRH), also known as somatocrinin or by several other names in its endogenous forms and as somatorelin (INN) in its pharmaceutical form, is a releasing hormone of growth hormone (GH). It is a 44-amino acid peptide hormone produced in the arcuate nucleus of the hypothalamus.

The beta-1 adrenergic receptor, also known as ADRB1, can refer to either the protein-encoding gene or one of the four adrenergic receptors. It is a G-protein coupled receptor associated with the Gs heterotrimeric G-protein that is expressed predominantly in cardiac tissue. In addition to cardiac tissue, beta-1 adrenergic receptors are also expressed in the cerebral cortex.

Heterotrimeric G protein, also sometimes referred to as the "large" G proteins are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of alpha (α), beta (β) and gamma (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein.

The Gs alpha subunit is a subunit of the heterotrimeric G protein Gs that stimulates the cAMP-dependent pathway by activating adenylyl cyclase. Gsα is a GTPase that functions as a cellular signaling protein. Gsα is the founding member of one of the four families of heterotrimeric G proteins, defined by the alpha subunits they contain: the Gαs family, Gαi/Gαo family, Gαq family, and Gα12/Gα13 family. The Gs-family has only two members: the other member is Golf, named for its predominant expression in the olfactory system. In humans, Gsα is encoded by the GNAS complex locus, while Golfα is encoded by the GNAL gene.

The bombesin receptor subtype 3 also known as BRS-3 or BB3 is a protein which in humans is encoded by the BRS3 gene.

The neuromedin B receptor (NMBR), now known as BB1 is a G protein-coupled receptor whose endogenous ligand is neuromedin B. In humans, this protein is encoded by the NMBR gene.

The gastrin-releasing peptide receptor (GRPR), now properly known as BB2 is a G protein-coupled receptor whose endogenous ligand is gastrin releasing peptide. In humans it is highly expressed in the pancreas and is also expressed in the stomach, adrenal cortex and brain.

cAMP-dependent protein kinase catalytic subunit beta is an enzyme that in humans is encoded by the PRKACB gene.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
Bombesin-like peptides comprise a large family of peptides which were initially isolated from amphibian skin, where they stimulate smooth muscle contraction. They were later found to be widely distributed in mammalian neural and endocrine cells.
The prostaglandin D2 (PGD2) receptors are G protein-coupled receptors that bind and are activated by prostaglandin D2. Also known as PTGDR or DP receptors, they are important for various functions of the nervous system and inflammation. They include the following proteins: