Related Research Articles

The paraventricular nucleus is a nucleus in the hypothalamus. Anatomically, it is adjacent to the third ventricle and many of its neurons project to the posterior pituitary. These projecting neurons secrete oxytocin and a smaller amount of vasopressin, otherwise the nucleus also secretes corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH). CRH and TRH are secreted into the hypophyseal portal system and act on different targets neurons in the anterior pituitary. PVN is thought to mediate many diverse functions through these different hormones, including osmoregulation, appetite, and the response of the body to stress.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.
Dynorphins (Dyn) are a class of opioid peptides that arise from the precursor protein prodynorphin. When prodynorphin is cleaved during processing by proprotein convertase 2 (PC2), multiple active peptides are released: dynorphin A, dynorphin B, and α/β-neoendorphin. Depolarization of a neuron containing prodynorphin stimulates PC2 processing, which occurs within synaptic vesicles in the presynaptic terminal. Occasionally, prodynorphin is not fully processed, leading to the release of “big dynorphin.” “Big Dynorphin” is a 32-amino acid molecule consisting of both dynorphin A and dynorphin B.

Ghrelin is a hormone primarily produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are highest before meals when hungry, returning to lower levels after mealtimes. Ghrelin may help prepare for food intake by increasing gastric motility and stimulating the secretion of gastric acid.

Neuropeptide Y (NPY) is a 36 amino-acid neuropeptide that is involved in various physiological and homeostatic processes in both the central and peripheral nervous systems. It is secreted alongside other neurotransmitters such as GABA and glutamate.
Bombesin is a 14-amino acid peptide originally isolated from the skin of the European fire-bellied toad by Vittorio Erspamer et al. and named after its source. It has two known homologs in mammals called neuromedin B and gastrin-releasing peptide. It stimulates gastrin release from G cells. It activates three different G-protein-coupled receptors known as BBR1, -2, and -3. It also activates these receptors in the brain. Together with cholecystokinin, it is the second major source of negative feedback signals that stop eating behaviour.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Calcitonin gene-related peptide (CGRP) is a member of the calcitonin family of peptides consisting of calcitonin, amylin, adrenomedullin, adrenomedullin 2 (intermedin) and calcitonin‑receptor‑stimulating peptide. Calcitonin is mainly produced by thyroid C cells whilst CGRP is secreted and stored in the nervous system. This peptide, in humans, exists in two forms: CGRP alpha, and CGRP beta. α-CGRP is a 37-amino acid neuropeptide and is formed by alternative splicing of the calcitonin/CGRP gene located on chromosome 11. β-CGRP is less studied. In humans, β-CGRP differs from α-CGRP by three amino acids and is encoded in a separate, nearby gene. The CGRP family includes calcitonin (CT), adrenomedullin (AM), and amylin (AMY).

Neurotensin is a 13 amino acid neuropeptide that is implicated in the regulation of luteinizing hormone and prolactin release and has significant interaction with the dopaminergic system. Neurotensin was first isolated from extracts of bovine hypothalamus based on its ability to cause a visible vasodilation in the exposed cutaneous regions of anesthetized rats.

Galanin is a neuropeptide encoded by the GAL gene, that is widely expressed in the brain, spinal cord, and gut of humans as well as other mammals. Galanin signaling occurs through three G protein-coupled receptors.

The lateral hypothalamus (LH), also called the lateral hypothalamic area (LHA), contains the primary orexinergic nucleus within the hypothalamus that widely projects throughout the nervous system; this system of neurons mediates an array of cognitive and physical processes, such as promoting feeding behavior and arousal, reducing pain perception, and regulating body temperature, digestive functions, and blood pressure, among many others. Clinically significant disorders that involve dysfunctions of the orexinergic projection system include narcolepsy, motility disorders or functional gastrointestinal disorders involving visceral hypersensitivity, and eating disorders.
Nesfatin-1 is a neuropeptide produced in the hypothalamus of mammals. It participates in the regulation of hunger and fat storage. Increased nesfatin-1 in the hypothalamus contributes to diminished hunger, a 'sense of fullness', and a potential loss of body fat and weight.

Neurokinin A (NKA), formerly known as Substance K, is a neurologically active peptide translated from the pre-protachykinin gene. Neurokinin A has many excitatory effects on mammalian nervous systems and is also influential on the mammalian inflammatory and pain responses.

Neuromedin N is a neuropeptide derived from the same precursor polypeptide as neurotensin, and with similar but subtly distinct expression and effects. Composed of the amino acid sequence Lys-Ile-Pro-Tyr-Ile-Leu, neuromedin N is homologous to neurotensin, both of whose sequences are found on the pro neurotensin/neuromedin N precursor C-terminus. Both sequences of neuromedin N as well as neurotensin are flanked by Lys-Arg amino acids, which comprise a consensus sequence for the endoprotease proprotein convertase. Neuromedin N is primarily synthesized in the neural and intestinal tissues of mammals; in studies performed in mice, neuromedin N's physiological effects were shown to include hypothermia and analgesia, arising from the peptide's ligand association to and interaction with neurotensin type 2 (NTS2) G protein-coupled receptors.

The urotensin-2 receptor (UR-II-R) also known as GPR14 is a class A rhodopsin family G protein coupled-receptor (GPCR) that is 386 amino acids long which binds primarily to the neuropeptide urotensin II.[1] The receptor quickly rose to prominence when it was found that when activated by urotensin II it induced the most potent vasoconstriction effect ever seen. While the precise function of the urotensin II receptor is not fully known it has been linked to cardiovascular effects, stress, and REM sleep.
The neuromedin U receptors are two G-protein coupled receptors which bind the neuropeptide hormones neuromedin U and neuromedin S. There are two subtypes of the neuromedin U receptor, each encoded by a separate gene.

Corticotropin-releasing hormone receptor 1 (CRHR1) is a protein, also known as CRF1, with the latter (CRF1) now being the IUPHAR-recommended name. In humans, CRF1 is encoded by the CRHR1 gene at region 17q21.31, beside micrototubule-associated protein tau MAPT.

Antalarmin (CP-156,181) is a drug that acts as a CRH1 antagonist.
Neuromedin S is a 36-amino acid neuropeptide found in the brain of humans and other mammals. It is produced in the suprachiasmatic nucleus of the hypothalamus and is related to neuromedin U. It is thought to be involved in regulation of circadian rhythm and also has appetite suppressant effects, as well as regulating the release of several other peptide hormones including vasopressin, luteinizing hormone, and oxytocin.
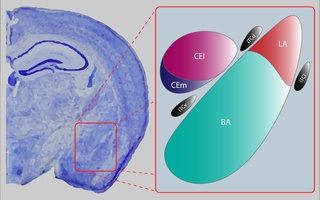
The central nucleus of the amygdala is a nucleus within the amygdala. It "serves as the major output nucleus of the amygdala and participates in receiving and processing pain information."
References
- 1 2 Brighton PJ, Szekeres PG, Willars GB (2004). "Neuromedin U and its receptors: structure, function, and physiological roles". Pharmacol. Rev. 56 (2): 231–48. doi:10.1124/pr.56.2.3. PMID 15169928. S2CID 884735.
- ↑ Torres R, Croll SD, Vercollone J, Reinhardt J, Griffiths J, Zabski S, Anderson KD, Adams NC, Gowen L, Sleeman MW, Valenzuela DM, Wiegand SJ, Yancopoulos GD, Murphy AJ (August 2007). "Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1, have impaired nociceptive responses". Pain. 130 (3): 267–78. doi:10.1016/j.pain.2007.01.036. PMID 17379411. S2CID 23617159.
- ↑ Novak CM, Zhang M, Levine JA (September 2007). "Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity". Brain Research. 1169: 57–68. doi:10.1016/j.brainres.2007.06.055. PMC 2735201 . PMID 17706946.
- ↑ Vigo E, Roa J, Pineda R, Castellano JM, Navarro VM, Aguilar E, Pinilla L, Tena-Sempere M (November 2007). "Novel role of the anorexigenic peptide neuromedin U in the control of LH secretion and its regulation by gonadal hormones and photoperiod". American Journal of Physiology. Endocrinology and Metabolism. 293 (5): E1265–73. doi:10.1152/ajpendo.00425.2007. PMID 17726140.
- ↑ Iwai T, Iinuma Y, Kodani R, Oka J (May 2008). "Neuromedin U inhibits inflammation-mediated memory impairment and neuronal cell-death in rodents". Neuroscience Research. 61 (1): 113–9. doi:10.1016/j.neures.2008.01.018. PMID 18336945. S2CID 25260405.
- ↑ Brighton PJ, Wise A, Dass NB, Willars GB (April 2008). "Paradoxical behavior of neuromedin U in isolated smooth muscle cells and intact tissue". The Journal of Pharmacology and Experimental Therapeutics. 325 (1): 154–64. doi:10.1124/jpet.107.132803. PMID 18180374. S2CID 33931881.
- ↑ Tanida M, Satomi J, Shen J, Nagai K (February 2009). "Autonomic and cardiovascular effects of central neuromedin U in rats". Physiology & Behavior. 96 (2): 282–8. doi:10.1016/j.physbeh.2008.10.008. PMID 18977236. S2CID 38382442.
- ↑ Mitchell JD, Maguire JJ, Kuc RE, Davenport AP (February 2009). "Expression and vasoconstrictor function of anorexigenic peptides neuromedin U-25 and S in the human cardiovascular system". Cardiovascular Research. 81 (2): 353–61. doi: 10.1093/cvr/cvn302 . PMID 18987052.
- 1 2 Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, Wu LJ, Toyoda H, Zhao MG, Rohde AD, Gragerova G, Onrust R, Bergmann JE, Zhuo M, Gaitanaris GA (December 2006). "Neuromedin U Receptor 2-Deficient Mice Display Differential Responses in Sensory Perception, Stress, and Feeding". Mol. Cell. Biol. 26 (24): 9352–63. doi:10.1128/MCB.01148-06. PMC 1698522 . PMID 17030627.
- 1 2 Ketterer K, Kong B, Frank D, Giese NA, Bauer A, Hoheisel J, Korc M, Kleeff J, Michalski CW, Friess H (May 2009). "Neuromedin U is overexpressed in pancreatic cancer and increases invasiveness via the hepatocyte growth factor c-Met pathway". Cancer Letters. 277 (1): 72–81. doi:10.1016/j.canlet.2008.11.028. PMID 19118941.
- 1 2 Brighton PJ, Szekeres PG, Wise A, Willars GB (December 2004). "Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Galphaq/11 and Galphai and an irreversible ligand-receptor interaction". Molecular Pharmacology. 66 (6): 1544–56. doi:10.1124/mol.104.002337. PMID 15331768. S2CID 25349752.