Endorphins are endogenous opioid neuropeptides and peptide hormones in humans and other animals. They are produced and stored in the pituitary gland. The classification of molecules as endorphins is based on their pharmacological activity, as opposed to a specific chemical formulation.

Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, suppressing cough, as well as for executions in the United States. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal.
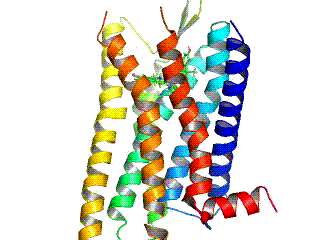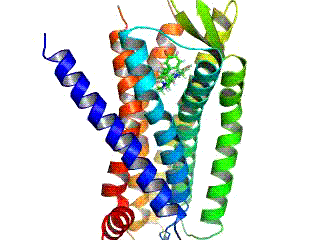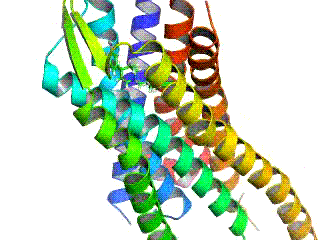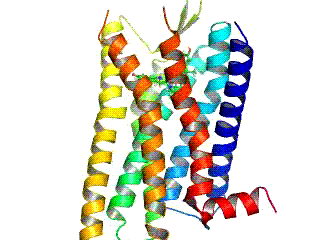
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.

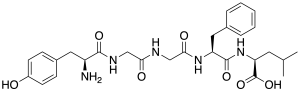
An enkephalin is a pentapeptide involved in regulating nociception in the body. The enkephalins are termed endogenous ligands, as they are internally derived and bind to the body's opioid receptors. Discovered in 1975, two forms of enkephalin have been found, one containing leucine ("leu"), and the other containing methionine ("met"). Both are products of the proenkephalin gene.

Beta-Endorphin or β-Endorphin is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Opioid peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, and the control of food intake.

Nociceptin/orphanin FQ (N/OFQ), a 17-amino acid neuropeptide, is the endogenous ligand for the nociceptin receptor, and initiates its function to act on numerous brain activities such as pain sensation and fear learning. It is derived from the prepronociceptin protein, as are a further 2 peptides, nocistatin & NocII, which inhibit the N/OFQ receptor function. Nociceptin itself acts as a potent anti-analgesic, effectively counteracting the effect of pain-relievers. The gene coding for prepronociceptin is located on Ch8p21 in humans. Nociceptin acts at the Nociceptin receptor formerly known as ORL1. Nociceptin is the first example of reverse pharmacology; the NOP receptor was discovered before the endogenous ligand which was discovered by two separate groups in 1995.

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.

SNC-80 is an opioid analgesic compound that selectively activates μ–δ opioid receptor heteromers and is used in scientific research. It was discovered in 1994.
DAMGO is a synthetic opioid peptide with high μ-opioid receptor specificity. It was synthesized as a biologically stable analog of δ-opioid receptor-preferring endogenous opioids, leu- and met-enkephalin. The crystal structure of DAMGO bound to the µ opioid receptor reveals a very similar binding pose to morphinans.

RB-101 is a drug that acts as an enkephalinase inhibitor, which is used in scientific research.
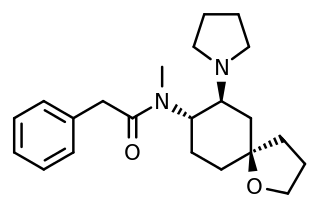
U-69,593 is a drug which acts as a potent and selective κ1-opioid receptor agonist. In animal studies it has been shown to produce antinociception, anti-inflammation, anxiolysis, respiratory depression, and diuresis, while having little effect on gastrointestinal motility. It also inhibits the peripheral, though not central secretion of oxytocin and vasopressin in rats.

Proenkephalin (PENK), formerly known as proenkephalin A, is an endogenous opioid polypeptide hormone which, via proteolyic cleavage, produces the enkephalin peptides [Met]enkephalin, and to a lesser extent, [Leu]enkephalin. Upon cleavage, each proenkephalin peptide results in the generation of four copies of [Met]enkephalin, two extended copies of [Met]enkephalin, and one copy of [Leu]enkephalin. Contrarily, [Leu]enkephalin] is predominantly synthesized from prodynorphin, which produces three copies of it per cleavage, and no copies of [Met]enkephalin. Other endogenous opioid peptides produced by proenkephalin include adrenorphin, amidorphin, BAM-18, BAM-20P, BAM-22P, peptide B, peptide E, and peptide F.

Hemorphin-4 is an endogenous opioid peptide of the hemorphin family which possesses antinociceptive properties and is derived from the β-chain of hemoglobin in the bloodstream. It is a tetrapeptide with the amino acid sequence Tyr-Pro-Trp-Thr. Hemorphin-4 has affinities for the μ-, δ-, and κ-opioid receptors that are in the same range as the structurally related β-casomorphins, although affinity to the κ-opioid receptor is markedly higher in comparison. It acts as an agonist at these sites. Hemorphin-4 also has inhibitory effects on angiotensin-converting enzyme (ACE), and as a result, may play a role in the regulation of blood pressure. Notably, inhibition of ACE also reduces enkephalin catabolism.
Leumorphin, also known as dynorphin B1–29, is a naturally occurring endogenous opioid peptide. Derived as a proteolytic cleavage product of residues 226-254 of prodynorphin, leumorphin is a nonacosapeptide and has the sequence Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr-Arg-Ser-Gln-Glu-Asp-Pro-Asn-Ala-Tyr-Ser-Gly-Glu-Leu-Phe-Asp-Ala. It can be further reduced to dynorphin B and dynorphin B-14 by pitrilysin metallopeptidase 1, an enzyme of the endopeptidase family. Leumorphin behaves as a potent and selective κ-opioid receptor agonist, similarly to other endogenous opioid peptide derivatives of prodynorphin.
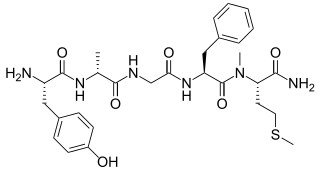
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine. It is an analgesic being more potent than morphine and about as potent as enkephalin.