
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it was not reported to exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.

Diphenoxylate is a centrally active opioid drug of the phenylpiperidine series that is used as a combination drug with atropine for the treatment of diarrhea. Diphenoxylate is an opioid and acts by slowing intestinal contractions; the atropine is present to prevent drug abuse and overdose. It should not be given to children due to the risk that they will stop breathing and should not be used in people with Clostridium difficile infection.

Ohmefentanyl is an extremely potent opioid analgesic drug which selectively binds to the µ-opioid receptor.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

N-Phenethyl-4-piperidinone (NPP) is a derivative of 4-piperidinone with the molecular formula C13H17NO. It is used as an intermediate in the manufacture of chemicals and pharmaceutical drugs such as fentanyl.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.
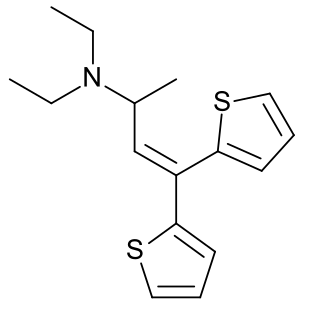
Diethylthiambutene is an opioid analgesic drug developed in the 1950s which was mainly used as an anesthetic in veterinary medicine and continues, along with the other two thiambutenes dimethylthiambutene and ethylmethylthiambutene to be used for this purpose, particularly in Japan. It is now under international control under Schedule I of the UN Single Convention On Narcotic Drugs 1961, presumably due to high abuse potential, although little more information is available. It is listed under Schedule I of the US Controlled Substances Act as a Narcotic and has an ACSCN of 9616 with zero annual manufacturing quota as of 2013.

Prodine is an opioid analgesic that is an analog of pethidine (meperidine). It was developed in Germany in the late 1940s.
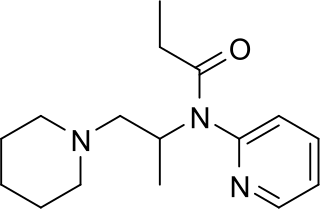
Propiram is a partial mu opioid receptor agonist and weak mu antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Metofoline (INN), also known as methofoline (USAN), is an opioid analgesic drug discovered in the 1950s by a team of Swiss researchers at Hoffmann-La Roche.
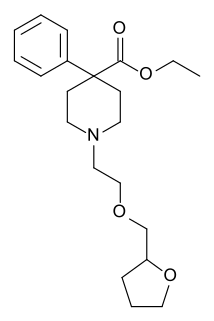
Furethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine), but with around 25x higher potency.

Mirfentanil is a fentanyl derivative with strong selectivity for the μ opioid receptor. At lower doses, it antagonizes the analgesic effects of alfentanil and substitutes for naloxone in morphine-treated monkeys; however, it also reverses naloxone-precipitated withdrawal in pigeons trained to discriminate morphine from naloxone.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.

Nalmexone (INN), or nalmexone hydrochloride (USAN), is a semisynthetic, opioid partial agonist or mixed agonist-antagonist with both analgesic and narcotic antagonist properties that was never marketed. In clinical studies it was found to have comparable analgesic efficacy to morphine, though with several-fold reduced potency. In addition, nalmexone's side effects, the most common of which were sleepiness and sweating, were reported to be similar to those of morphine, albeit with a noticeably higher degree of incidence.

U-47700, also known as pink heroin, pinky, and pink, is an opioid analgesic drug developed by a team at Upjohn in the 1970s which has around 7.5 times the potency of morphine in animal models.

3-Methylbutyrfentanyl (3-MBF) is an opioid analgesic that is an analog of butyrfentanyl.

OPPPP is an opioid drug related to medicines such as prodine. It is one of several compounds derived from MPPP, the reverse ester of pethidine, which were sold as designer drugs in the 1980s, but have been rarely encountered by law enforcement since the passage of the Federal Analogue Act in 1986. In animal studies it was found to be around 1000x the potency of pethidine, making it several times the potency of fentanyl and with similar hazards of respiratory depression and overdose. It is closely related to numerous compounds made by Janssen et al. for which the structure-activity relationship is well established.



















