| |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H25NO |
| Molar mass | 247.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
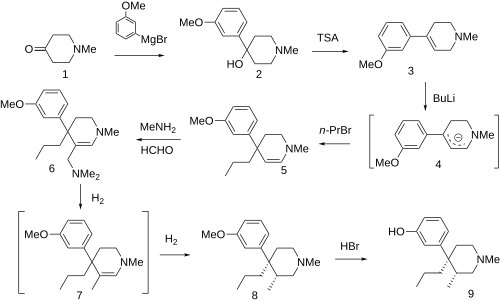
Picenadol (LY-97435) is a 4-phenyl piperidine derivative that is an opioid analgesic drug developed by Eli Lilly in the 1970s. [1]
Contents
Picenadol is an effective analgesic with similar efficacy to pethidine (meperidine). It has been investigated for some applications such as obstetrics [2] and dentistry, [3] but never commercialised.
It is unusual in that one enantiomer is a pure μ-opioid agonist, while the other is an antagonist. [4] The (3R,4R) isomer is the agonist, while (3S,4S) is antagonist. [5] This means that the racemic mix of the two enantiomers is a mixed agonist-antagonist, with relatively low abuse potential, and little of the κ-opioid activity that tends to cause problems with other opioid mixed agonist-antagonists such as pentazocine. [6]