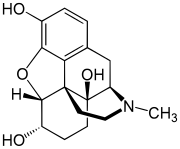
Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, Thēbai (Thebes), an ancient city in Upper Egypt. A minor constituent of opium, thebaine is chemically similar to both morphine and codeine, but has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)-thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine. While thebaine is not used therapeutically, it is the main alkaloid extracted from Papaver bracteatum and can be converted industrially into a variety of compounds, including hydrocodone, hydromorphone, oxycodone, oxymorphone, nalbuphine, naloxone, naltrexone, buprenorphine, butorphanol and etorphine.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.
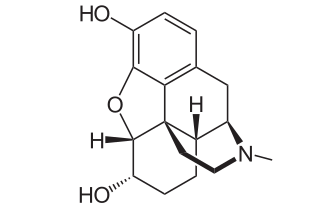
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine with a side effect profile similar to that of dihydromorphine, morphine, and diamorphine.

Nicocodeine is an opioid analgesic and cough suppressant, an ester of codeine closely related to dihydrocodeine and the codeine analogue of nicomorphine. It is not commonly used in most countries, but has activity similar to other opiates. Nicocodeine and nicomorphine were synthesized in 1904, and introduced in 1957 by Lannacher Heilmittel of Austria. Nicocodeine is metabolised in the liver by demethylation to produce nicomorphine, also known as 6-nicotinoylmorphine, and subsequently further metabolised to morphine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression. Related opioid analogues such as nicomorphine and nicodicodeine were first synthesized. The definitive synthesis, which involves treating anhydrous codeine base with nicotinic anhydride at 130 °C, was published by Pongratz and Zirm in Monatshefte für Chemie in 1957, simultaneously with the two analogues in an article about amides and esters of various organic acids.

Acetyldihydrocodeine is an opiate derivative discovered in Germany in 1914 and was used as a cough suppressant and analgesic. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of thebacon, where only the 6-7 bond is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of dihydrocodeine and is metabolised in the liver by demethylation and deacetylation to produce dihydromorphine.

Metopon is an opioid analogue that is a methylated derivative of hydromorphone which was invented in 1929 as an analgesic.
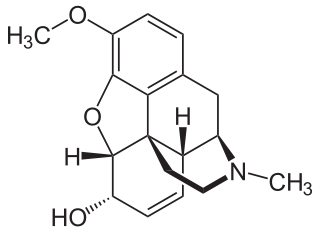
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of addiction and overdose.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.

Normethadone, also known as desmethylmethadone or phenyldimazone, is a synthetic opioid analgesic and antitussive agent. Normethadone is listed under the Single Convention on Narcotic Drugs 1961 and is a Schedule I Narcotic controlled substance in the United States, with a DEA ACSCN of 9635 and an annual manufacturing quota of 2 grams. It has an effective span of action for about 14 days, and is 12 to 20 times stronger than morphine. The salts in use are the hydrobromide, hydrochloride (0.890), methyliodide (0.675), oxalate (0.766), picrate (0.563), and the 2,6-ditertbutylnapthalindisulphonate (0.480).

Tilidine, sold under the brand name Valoron among others, is a synthetic opioid analgesic, used mainly in Belgium, Bulgaria, Germany, Albania, Luxembourg, South Africa, and Switzerland for the treatment of moderate to severe pain, both acute and chronic. Its onset of pain relief after oral administration is about 10–15 minutes and peak relief from pain occurs about 25–50 minutes after administration.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Phenampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and diampromide. It was invented in the 1960s by American Cyanamid Co. Although never given a general release, it was research found that 60 mg of phenampromide is equivalent to about 50 mg of codeine. Tests on its two enantiomers showed that all of the analgesic effects were caused by the (S)-isomer. Introduction of a phenyl group to the 4-position of the piperidine-ring produces a drug 60-fold more potent than morphine. The most potent reported derivative is 4-hydroxy-4-phenyl phenapromide which displays analgesic activity some x150 greater than morphine.

Difenoxin is an opioid drug used, often in combination with atropine, to treat diarrhea. It is the principal metabolite of diphenoxylate.

Myrophine (Myristylbenzylmorphine) is an opiate analogue that was developed in 1952. It is a derivative of morphine.

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.
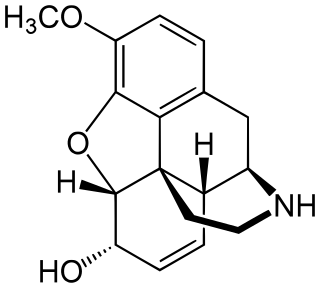
Norcodeine is an opiate analogue that is the N-demethylated derivative of codeine. It has relatively little opioid activity in its own right, but is formed as a metabolite of codeine following ingestion.

Methyldesorphine is an opioid analgesic. First synthesized in Germany in 1940 and patented in the US in 1952, it has a high potential for abuse as with any potent opioid agonist, and is sometimes found along with desomorphine as a component of the home-made opioid mixture known as "Krokodil" used in Russia and the neighboring former Soviet republics. It is approximately 15 times more potent than morphine as an analgesic but if the 6-7 bond is saturated, the β isomer is some 50 times more potent than morphine.

Codeine-N-oxide (genocodeine) is an active metabolite of codeine. It is an opiate listed as a Schedule I controlled substance. It has a DEA ACSCN of 9053 and its annual manufacturing quota for 2013 was 602 grams.