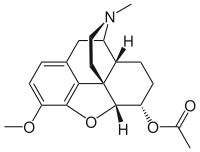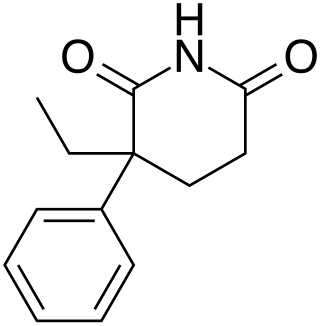
Glutethimide is a hypnotic sedative that was introduced by Ciba in 1954 as a safe alternative to barbiturates to treat insomnia. Before long, however, it had become clear that glutethimide was just as likely to cause addiction and caused similar withdrawal symptoms. Doriden was the brand-name version. Current production levels in the United States point to its use only in small-scale research. Manufacturing of the drug was discontinued in the US in 1993 and discontinued in several eastern European countries in 2006.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.
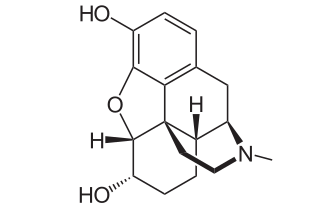
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Levomethorphan (LVM) (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine with a side effect profile similar to that of dihydromorphine, morphine, and diamorphine.

Nicocodeine is an opioid analgesic and cough suppressant, an ester of codeine closely related to dihydrocodeine and the codeine analogue of nicomorphine. It is not commonly used in most countries, but has activity similar to other opiates. Nicocodeine and nicomorphine were synthesized in 1904, and introduced in 1957 by Lannacher Heilmittel of Austria. Nicocodeine is metabolised in the liver by demethylation to produce nicomorphine, also known as 6-nicotinoylmorphine, and subsequently further metabolised to morphine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression. Related opioid analogues such as nicomorphine and nicodicodeine were first synthesized. The definitive synthesis, which involves treating anhydrous codeine base with nicotinic anhydride at 130 °C, was published by Pongratz and Zirm in Monatshefte für Chemie in 1957, simultaneously with the two analogues in an article about amides and esters of various organic acids.

Nicodicodine is an opioid developed as a cough suppressant and analgesic. Synthesized in 1904, it is not commonly used, but has activity similar to other opioids. Nicodicodine is metabolised in the liver by demethylation to produce 6-nicotinoyldihydromorphine, and subsequently further metabolised to dihydromorphine. Since the final active metabolite is the slightly stronger opiate dihydromorphine rather than morphine, nicodicodine can be expected to be marginally more potent and longer acting than nicocodeine. Side effects are similar to those of other opioids and include itching, nausea and respiratory depression.

Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of addiction and overdose.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.

Phenomorphan is an opioid analgesic. It is not currently used in medicine, but has similar side-effects to other opiates, which include itching, nausea and respiratory depression.

Allylprodine is an opioid analgesic that is an analog of prodine. It was discovered by Hoffman-La Roche in 1957 during research into the related drug pethidine. Derivatives were tested to prove the theory that phenolic and non-phenolic opioids bind at different sites of the opiate receptor.

Phenampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and diampromide. It was invented in the 1960s by American Cyanamid Co. Although never given a general release, it was research found that 60 mg of phenampromide is equivalent to about 50 mg of codeine. Tests on its two enantiomers showed that all of the analgesic effects were caused by the (S)-isomer. Introduction of a phenyl group to the 4-position of the piperidine-ring produces a drug 60-fold more potent than morphine. The most potent reported derivative is 4-hydroxy-4-phenyl phenapromide which displays analgesic activity some x150 greater than morphine.

Drotebanol (Oxymethebanol) is a morphinan derivative that acts as an opioid agonist. It was invented by Sankyo Company in Japan during the 1970s. It is synthesised from thebaine.
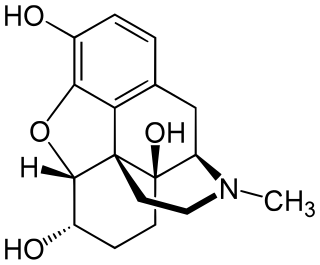
Hydromorphinol, is an opiate analogue that is a derivative of morphine, where the 14-position has been hydroxylated and the 7,8- double bond saturated. It has similar effects to morphine such as sedation, analgesia and respiratory depression, but is twice as potent as morphine and has a steeper dose-response curve and longer half-life. It is used in medicine as the bitartrate salt and hydrochloride
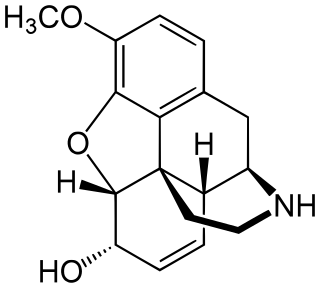
Norcodeine is an opiate analogue that is the N-demethylated derivative of codeine. It has relatively little opioid activity in its own right, but is formed as a metabolite of codeine following ingestion.

An opiate is an alkaloid substance derived from opium. It differs from the similar term opioid in that the latter is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

Morphine-N-oxide (genomorphine) is an active opioid metabolite of morphine. Morphine itself, in trials with rats, is 11–22 times more potent than morphine-N-oxide subcutaneously and 39–89 times more potent intraperitoneally. However, pretreatment with amiphenazole or tacrine increases the potency of morphine-N-oxide in relation to morphine. A possible explanation is that morphine-N-oxide is rapidly inactivated in the liver and impairment of inactivation processes or enzymes increases functionality.

Codeine-N-oxide (genocodeine) is an active metabolite of codeine. It is an opiate listed as a Schedule I controlled substance. It has a DEA ACSCN of 9053 and its annual manufacturing quota for 2013 was 602 grams.