 | |
| Clinical data | |
|---|---|
| Drug class | Opioid |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
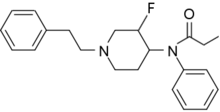
| Formula | C22H27FN2O |
| Molar mass | 354.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
NFEPP (N-(3-fluoro-1-phenethylpiperidin-4-yl)-N-phenylpropionamide) is an analgesic opioid chemical, similar in structure to fentanyl, designed in 2016 by Spahn et al. from Free University of Berlin [2] to avoid the standard negative side effects of opiates, including opioid overdose, by only targeting inflamed tissue. [3] [4]