Related Research Articles

Hydrocodone, also known as dihydrocodeinone, is a semisynthetic opioid used to treat pain and as a cough suppressant. It is taken by mouth. Typically it is dispensed as the combination acetaminophen/hydrocodone or ibuprofen/hydrocodone for pain severe enough to require an opioid and in combination with homatropine methylbromide to relieve cough. It is also available by itself in a long-acting form under the brand name Zohydro ER, among others, to treat severe pain of a prolonged duration. Hydrocodone is a controlled drug: in the United States a Schedule II Controlled Substance.
An antipyretic is a substance that reduces fever. Antipyretics cause the hypothalamus to override a prostaglandin-induced increase in temperature. The body then works to lower the temperature, which results in a reduction in fever.
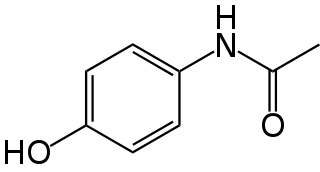
Paracetamol is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely used over the counter medication. Common brand names include Tylenol and Panadol.
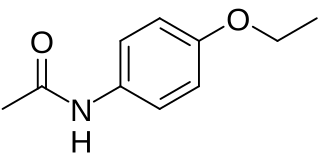
Phenacetin is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s.

Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a fully synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, in Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines, the prodines, bemidones and others more distant, including diphenoxylate and analogues.
ATC code A02Drugs for acid related disorders is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup A02 is part of the anatomical group A Alimentary tract and metabolism.
ATC code B01Antithrombotic agents is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup B01 is part of the anatomical group B Blood and blood forming organs.
ATC code C01Cardiac therapy is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup C01 is part of the anatomical group C Cardiovascular system.
ATC code M03Muscle relaxants is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup M03 is part of the anatomical group M Musculo-skeletal system.
ATC code R03Drugs for obstructive airway diseases is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup R03 is part of the anatomical group R Respiratory system.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration.

The Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP), also known as the Poisons Standard for short, is an Australian legislative instrument produced by the Therapeutic Goods Administration (TGA). Before 2010, it was known as the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP). The SUSMP classifies drugs and poisons into different Schedules signifying the degree of control recommended to be exercised over their availability to the public. As of 2023, the most recent version is the Therapeutic Goods Instrument 2023.

Analgesic nephropathy is injury to the kidneys caused by analgesic medications such as aspirin, bucetin, phenacetin, and paracetamol. The term usually refers to damage induced by excessive use of combinations of these medications, especially combinations that include phenacetin. It may also be used to describe kidney injury from any single analgesic medication.

Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children or adults. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.
"Pain ladder", or analgesic ladder, was created by the World Health Organization (WHO) as a guideline for the use of drugs in the management of pain. Originally published in 1986 for the management of cancer pain, it is now widely used by medical professionals for the management of all types of pain.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others.
The major drug laws of India are the Narcotic Drugs and Psychotropic Substances Act (1985) and the Prevention of Illicit Trafficking in Narcotic Drugs and Psychotropic Substances Act (1988).
The Brazilian Controlled Drugs and Substances Act, officially Portaria nº 344/1998, is Brazil's federal drug control statute, issued by the Ministry of Health through its National Health Surveillance Agency (Anvisa). The act also serves as the implementing legislation for the Single Convention on Narcotic Drugs, the Convention on Psychotropic Substances, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances in the country.
References
- ↑ "ATC (Anatomical Therapeutic Chemical Classification System) – Synopsis". National Institutes of Health . Retrieved 1 February 2020.
- ↑ World Health Organization. "Anatomical Therapeutic Chemical (ATC) Classification". World Health Organization. Retrieved 3 January 2022.
- ↑ "Structure and principles". WHO Collaborating Centre for Drug Statistics Methodology. 15 February 2018. Retrieved 3 January 2022.
- ↑ "ATC/DDD Index 2022: code N02". WHO Collaborating Centre for Drug Statistics Methodology.
- ↑ "ATCvet Index 2022: code QN02". WHO Collaborating Centre for Drug Statistics Methodology.