
An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABAA receptor agonists, however recently proton pump inhibitors, aromatase inhibitors, NSAIDs and other classes of drugs in this class have been developed as well. Despite usually being similar to them in effect, they are not chemically related to benzodiazepines. As such, GABAA-agonizing imidazopyridines, pyrazolopyrimidines, and cyclopyrrones are sometimes grouped together and referred to as "nonbenzodiazepines." Imidazopyridines include:

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).
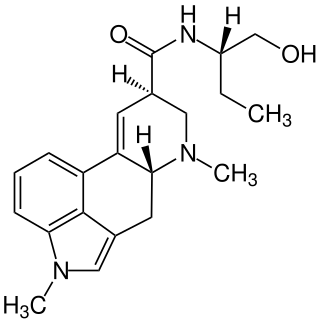
Methysergide, sold under the brand names Deseril and Sansert, is a monoaminergic medication of the ergoline and lysergamide groups which is used in the prophylaxis and treatment of migraine and cluster headaches. It has been withdrawn from the market in the United States and Canada due to adverse effects. It is taken by mouth.

meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.
Neurokinin 1 (NK1) antagonists (-pitants) are a novel class of medications that possesses unique antidepressant, anxiolytic, and antiemetic properties. NK-1 antagonists boost the efficacy of 5-HT3 antagonists to prevent nausea and vomiting. The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.

P2Y receptors are a family of purinergic G protein-coupled receptors, stimulated by nucleotides such as adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate and UDP-glucose.To date, 8 P2Y receptors have been cloned in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Neurotensin receptor type 2 is a protein that in humans is encoded by the NTSR2 gene.
A cholecystokinin receptor antagonist is a specific type of receptor antagonist which blocks the receptor sites for the peptide hormone cholecystokinin (CCK).
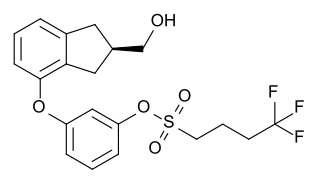
Originally synthesized by chemist Wayne E. Kenney, BAY 38-7271 (KN 38-7271) is a drug which is a cannabinoid receptor agonist developed by Bayer AG. It has analgesic and neuroprotective effects and is used in scientific research, with proposed uses in the treatment of traumatic brain injury. It is a full agonist with around the same potency as CP 55,940 in animal studies, and has fairly high affinity for both CB1 and CB2 receptors, with Ki values of 2.91nM at CB1 and 4.24nM at CB2. It has been licensed to KeyNeurotek Pharmaceuticals for clinical development, and was in Phase II trials in 2008 but its development appears to have stopped.
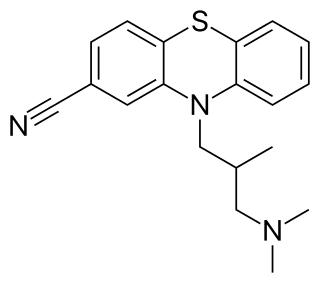
Cyamemazine (Tercian), also known as cyamepromazine, is a typical antipsychotic drug of the phenothiazine class which was introduced by Theraplix in France in 1972 and later in Portugal as well.

Rhynchophylline is an alkaloid found in certain Uncaria species (Rubiaceae), notably Uncaria rhynchophylla and Uncaria tomentosa. It also occurs in the leaves of Mitragyna speciosa (kratom), a tree native to Thailand. Chemically, it is related to the alkaloid mitragynine.

BIIE-0246 is a drug used in scientific research which acts as a potent and selective antagonist for the Neuropeptide Y receptor Y2. It was one of the first non-peptide Y2-selective antagonists developed, and remains among the most widely used tools for studying this receptor. It has been used to demonstrate a role for the Y2 subtype as a presynaptic autoreceptor limiting further neuropeptide Y release, as well as modulating dopamine and acetylcholine release. It has also been shown to produce several behavioural effects in animals, including reducing alcohol consumption in addicted rats and anxiolytic effects, although while selective Y2 agonists are expected to be useful as anorectics, BIIE-0246 did not appear to increase appetite when administered alone.

Eptapirone (F-11,440) is a very potent and highly selective 5-HT1A receptor full agonist of the azapirone family. Its affinity for the 5-HT1A receptor was reported to be 4.8 nM (Ki), and its intrinsic activity approximately equal to that of serotonin.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

Ro5-4864 (4'-chlorodiazepam) is a drug which is a benzodiazepine derivative of diazepam. However unlike most benzodiazepine derivatives, Ro5-4864 lacks affinity for GABAA receptors and lacks typical benzodiazepine effects, instead being sedative yet also convulsant and anxiogenic in effects. Ro5-4864 was found to be a potent ligand for the "peripheral benzodiazepine receptor", later renamed to mitochondrial translocator protein 18kDa (TSPO). Despite its convulsant effects, at lower doses Ro5-4864 has proved to be neuroprotective and has become widely used for research into the role of the TSPO protein in neurotoxicity. In vitro studies and rodent models also suggest the possibility of analgesic, antidepressant, cardioprotective, and anti-cancer effects.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

Lomerizine (INN) is a diphenylpiperazine class L-type and T-type calcium channel blocker. This drug is currently used clinically for the treatment of migraines, while also being used experimentally for the treatment of glaucoma and optic nerve injury.

CP-122,288 is a drug which acts as a potent and selective agonist for the 5-HT1B, 5-HT1D and 5-HT1F serotonin receptor subtypes. It is a derivative of the migraine medication sumatriptan, but while CP-122,288 is 40,000 times more potent than sumatriptan as an inhibitor of neurogenic inflammation and plasma protein extravasation, it is only twice as potent as a constrictor of blood vessels. In human trials, CP-122,288 was not found to be effective as a treatment for migraine, but its selectivity for neurogenic anti-inflammatory action over vasoconstriction has made it useful for research into the underlying causes of migraine.


















