The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

YM-348 is an indazole derivative drug which acts as a potent and selective 5-HT2C receptor agonist, with an EC50 of 1nM and 15x selectivity over 5-HT2A, although it only has moderate selectivity of 3x over the closely related 5-HT2B receptor. It has thermogenic and anorectic effects in animal studies, making it potentially useful for the treatment of obesity.
AL-38022A is an indazole derivative drug which is one of a range of similar drugs developed for scientific research and with some possible clinical applications. It acts as a potent and selective agonist for the 5-HT2 family of serotonin receptors, with highest binding affinity for the 5-HT2C subtype and around four times less affinity for 5-HT2A and 5-HT2B. In drug discrimination tests on animals, it fully substituted for both DOM and 5-MeO-DMT.
α-Methylserotonin (αMS), also known as α-methyl-5-hydroxytryptamine (α-methyl-5-HT) or 5-hydroxy-α-methyltryptamine (5-HO-αMT), is a tryptamine derivative closely related to the neurotransmitter serotonin (5-HT). It acts as a non-selective serotonin receptor agonist and has been used extensively in scientific research to study the function of the serotonin system.

AL-37350A (4,5-DHP-AMT) is a tricyclic tryptamine derivative which acts as a potent and selective agonist for the serotonin receptor 5-HT2A, with a Ki of 2.0 nM, and moderate selectivity over the related 5-HT2B and 5-HT2C receptors. It has been shown to have ocular hypotensive activity in animal models, suggesting it may be useful for the treatment of glaucoma.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. Most compounds of this class are potent and long-lasting psychedelic drugs, and act as highly selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonists. A few bulkier derivatives such as DOAM have similarly high binding affinity for 5-HT2 receptors but instead act as antagonists, and so do not produce psychedelic effects though they retain amphetamine-like stimulant effects.

VER-3323 is a drug which acts as a selective agonist for both the 5-HT2B and 5-HT2C serotonin receptor subtypes, with moderate selectivity for 5-HT2C, but relatively low affinity for 5-HT2A. It has potent anorectic effects in animal studies.
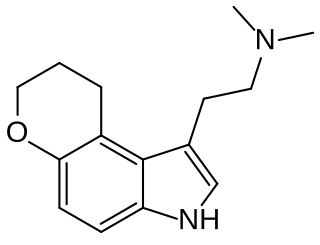
1-(2-Dimethylaminoethyl)dihydropyrano(3,2-e)indole (4,5-DHP-DMT) is a tricyclic tryptamine derivative which acts as a potent and reasonably selective partial agonist for the serotonin receptor 5-HT2A, with a Ki of 17.0 nM, and moderate selectivity over related serotonin receptors. It has lower 5-HT2 affinity and efficacy than the related compound AL-37350A, but higher lipophilicity.

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.
5-HT2C receptor agonists are a class of drugs that activate 5-HT2C receptors. They have been investigated for the treatment of a number of conditions including obesity, psychiatric disorders, sexual dysfunction and urinary incontinence.

O-4310 (1-isopropyl-6-fluoro-psilocin) is a tryptamine derivative developed by Organix Inc which acts as a serotonin receptor agonist. It is claimed to have an EC50 of 5 nM at the 5-HT2A receptor with 89% efficacy relative to serotonin, and 100-fold selectivity over the 5-HT2C receptor, while being apparently inactive at the 5-HT2B antitarget.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

CP-132,484 is a tryptamine derivative which acts as a potent and selective agonist for the 5-HT2 family of serotonin receptors. It has reasonable selectivity for 5-HT2A and 5-HT2C subtypes over 5-HT2B, but is only slightly selective for 5-HT2A over 5-HT2C. This compound and several related analogues have been shown to have ocular hypotensive activity in animal models, suggesting they may be useful for the treatment of glaucoma.

SN-22 is a chemical compound which acts as a moderately selective agonist at the 5-HT2 family of serotonin receptors, with a Ki of 19 nM at 5-HT2 subtypes versus 514 nM at 5-HT1A receptors. Many related derivatives are known, most of which are ligands for 5-HT1A, 5-HT6 or dopamine D2 receptors or show SSRI activity.
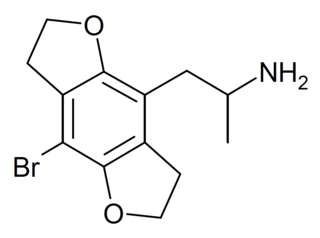
DOB-FLY is a recreational designer drug with psychedelic effects. It can be regarded as the alpha-methyl derivative of 2C-B-FLY or the partially saturated counterpart of bromo-dragonfly. Unlike bromo-dragonfly, DOB-FLY is only slightly more potent than DOB itself, with an active dose in humans of around 1 mg.

SCHEMBL5334361 is a drug which acts as an agonist at the 5-HT2 family of serotonin receptors, and was developed for the treatment of glaucoma. It is a benzazepine derivative structurally related to the anorectic drug lorcaserin. It is selective for 5-HT2A, with an EC50 of 0.4nM at 5-HT2A vs 3.9nM at 5-HT2C and a much lower affinity of 417nM at 5-HT2B.

ZC-B (3-(4-bromo-2,5-dimethoxyphenyl)azetidine) is a phenethylamine derivative which acts as a serotonin receptor agonist selective for the 5-HT2 subtypes, with an EC50 of 1.6nM at 5-HT2A, vs 5.8nM at 5-HT2C. It is related to psychedelic phenethylamine derivatives such as 2C-B and mescaline but with the ethylamine side chain replaced by an azetidine ring.

VU6067416 is an indazole derivative which acts as an agonist for the 5-HT2 family of serotonin receptors. It is a potent full agonist at 5-HT2B, and has slightly lower affinity and partial agonist effects at 5-HT2A and 5-HT2C, though some related compounds have improved selectivity for 5-HT2A.