
N,N-Dimethyltryptamine is a substituted tryptamine that occurs in many plants and animals, including humans, and which is both a derivative and a structural analog of tryptamine. DMT is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.

Lysergic acid diethylamide, commonly known as LSD, and known colloquially as acid or lucy, is a potent psychedelic drug. Effects typically include intensified thoughts, emotions, and sensory perception. At sufficiently high dosages, LSD manifests primarily mental, visual, and auditory hallucinations. Dilated pupils, increased blood pressure, and increased body temperature are typical.

Psychopharmacology is the scientific study of the effects drugs have on mood, sensation, thinking, behavior, judgment and evaluation, and memory. It is distinguished from neuropsychopharmacology, which emphasizes the correlation between drug-induced changes in the functioning of cells in the nervous system and changes in consciousness and behavior.
Neuropsychopharmacology, an interdisciplinary science related to psychopharmacology and fundamental neuroscience, is the study of the neural mechanisms that drugs act upon to influence behavior. It entails research of mechanisms of neuropathology, pharmacodynamics, psychiatric illness, and states of consciousness. These studies are instigated at the detailed level involving neurotransmission/receptor activity, bio-chemical processes, and neural circuitry. Neuropsychopharmacology supersedes psychopharmacology in the areas of "how" and "why", and additionally addresses other issues of brain function. Accordingly, the clinical aspect of the field includes psychiatric (psychoactive) as well as neurologic (non-psychoactive) pharmacology-based treatments. Developments in neuropsychopharmacology may directly impact the studies of anxiety disorders, affective disorders, psychotic disorders, degenerative disorders, eating behavior, and sleep behavior.

Cycloserine, sold under the brand name Seromycin, is a GABA transaminase inhibitor and an antibiotic, used to treat tuberculosis. Specifically it is used, along with other antituberculosis medications, for active drug resistant tuberculosis. It is given by mouth.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

25I-NBOMe, also known as Smiles, or N-Bomb, is a novel synthetic psychoactive substance with strong hallucinogenic properties, synthesized in 2003 for research purposes. Since 2010, it has circulated in the recreational drug scene, often misrepresented as LSD.
Hallucinogens are a large and diverse class of psychoactive drugs that can produce altered states of consciousness characterized by major alterations in thought, mood, and perception as well as other changes. Most hallucinogens can be categorized as either being psychedelics, dissociatives, or deliriants.

AL-34662 is an indazole derivative drug that is being developed for the treatment of glaucoma. It acts as a selective 5-HT2A receptor agonist, the same target as that of psychedelic drugs like psilocin, but unlike these drugs, AL-34662 was designed specifically as a peripherally selective drug, which does not cross the blood–brain barrier. This means that AL-34662 can exploit a useful side effect of the hallucinogenic 5-HT2A agonists, namely reduction in intra-ocular pressure and hence relief from the symptoms of glaucoma, but without causing the hallucinogenic effects that make centrally active 5-HT2A agonists unsuitable for clinical use. In animal studies, AL-34662 has been shown to be potent and effective in the treatment of symptoms of glaucoma, with minimal side effects.
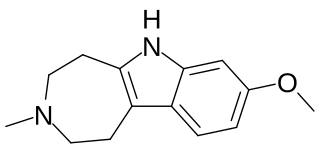
Tabernanthalog (TBG) is a novel water-soluble, non-toxic azepinoindole analog of the psychoactive drug ibogaine first synthesized by Professor David E. Olson at UC Davis.
Psychoplastogens are a group of small molecule drugs that produce rapid and sustained effects on neuronal structure and function, intended to manifest therapeutic benefit after a single administration. Several existing psychoplastogens have been identified and their therapeutic effects demonstrated; several are presently at various stages of development as medications including ketamine, MDMA, scopolamine, and the serotonergic psychedelics, including LSD, psilocin, DMT, and 5-MeO-DMT. Compounds of this sort are being explored as therapeutics for a variety of brain disorders including depression, addiction, and PTSD. The ability to rapidly promote neuronal changes via mechanisms of neuroplasticity was recently discovered as the common therapeutic activity and mechanism of action.
David E. Olson is an American chemist and neuroscientist. He is an associate professor of chemistry, biochemistry and molecular medicine at the University of California, Davis, and is the founding director of the UC Davis Institute for Psychedelics and Neurotherapeutics.
Delix Therapeutics is an American biotech company based in Boston, Massachusetts. The company develops novel neuroplasticity-promoting therapeutics for central nervous system (CNS) diseases such as depression and post-traumatic stress disorder (PTSD). It was co-founded in 2019 by David E. Olson and Nick Haft.
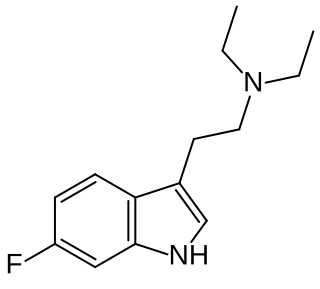
6-Fluoro-DET is a substituted tryptamine derivative related to drugs such as DET and 5-fluoro-DET. It acts as a partial agonist at the 5-HT2A receptor, but while it produces similar physiological effects to psychedelic drugs, it does not appear to produce psychedelic effects itself even at high doses. For this reason it saw some use as an active placebo in early clinical trials of psychedelic drugs but was regarded as having little use otherwise, though more recent research into compounds such as AL-34662, TBG and AAZ-A-154 has shown that these kind of non-psychedelic 5-HT2A agonists can have various useful applications.
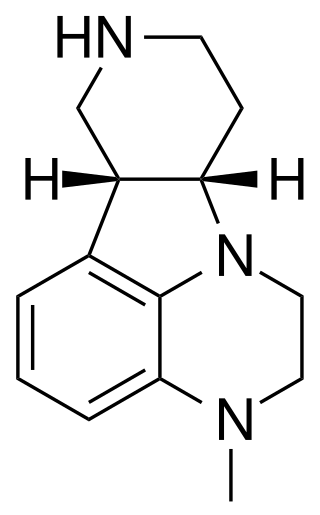
IHCH-7113 is a drug which acts as an agonist at the 5-HT2A serotonin receptor. It was derived by structural simplification of the 5-HT2A antagonist atypical antipsychotic drug lumateperone along with several related compounds such as IHCH-7079 and IHCH-7086, which were found to be nonhallucinogenic biased 5-HT2A agonists that were active in antidepressant assays but did not produce psychedelic-like responding in mice. IHCH-7113 however produced a head-twitch response comparable to that of DOI or LSD, which was blocked by the 5-HT2A antagonist MDL100907.
Lin Tian is a Chinese-American neuroscientist and biochemist. She is a Scientific Director of the Max Planck Florida Institute for Neuroscience in Jupiter, FL, and was formerly a professor in the Department of Biochemistry and Molecular Medicine at the University of California, Davis. Tian is known for her research in the fields of neuroscience and biochemical engineering. She develops and applies molecular tools to understand brain function and dysfunction at the individual, neuronal level.
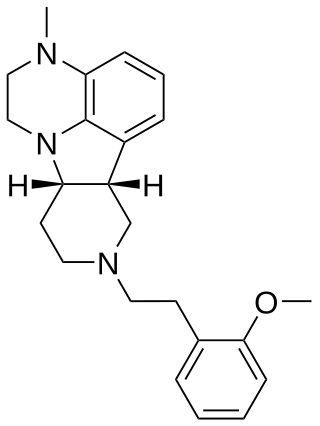
IHCH-7079 is a drug which acts as an agonist at the 5-HT2A serotonin receptor. It was derived by structural simplification of the 5-HT2A antagonist atypical antipsychotic drug lumateperone along with several related compounds such as IHCH-7086, which was found to be a non-hallucinogenic biased 5-HT2A agonist that was active in antidepressant assays but did not produce psychedelic-like responding in mice. The related structure IHCH-7113 was found to produce a positive head-twitch response in mice, suggesting likely hallucinogenic activity in humans.

IHCH-7086 is a drug which acts as an agonist at the 5-HT2A serotonin receptor. It was derived by structural simplification of the 5-HT2A antagonist atypical antipsychotic drug lumateperone along with several related compounds such as IHCH-7079, which was found to be a non-hallucinogenic biased 5-HT2A agonist that was active in antidepressant assays but did not produce psychedelic-like responding in mice. The related structure IHCH-7113 was found to produce a positive head-twitch response in mice, suggesting likely hallucinogenic activity in humans.













