
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the mu opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor. 5-HT is short for 5-hydroxy-tryptamine, which is serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones.

The κ-opioid receptor, abbreviated KOR or KOP, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin) receptors which are G protein-coupled receptors. This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems.
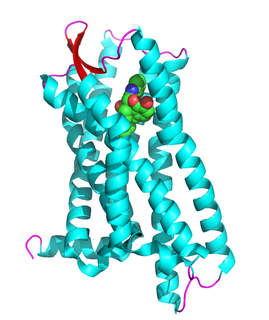
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.
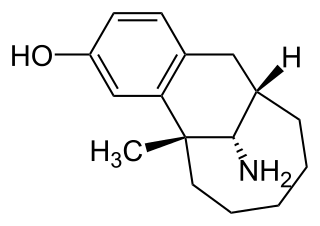
Dezocine is a marketed opioid analgesic of the benzomorphan group. First synthesized in 1970, it acts as a modulator of mu-, delta-, and kappa-opioid receptors. Dezocine is a mixed agonist/antagonist of opioid receptors. It is related to other benzomorphans such as pentazocine, with a similar profile of effects that include analgesia and euphoria. Unlike many other benzomorphans however, it is a silent antagonist of the κ-opioid receptor, and in accordance, does not produce side effects such as dysphoria or hallucinations at any dose.
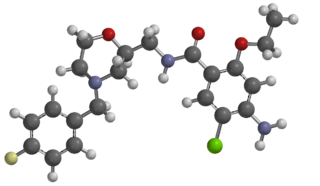
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).

5-Hydroxytryptamine receptor 4 is a protein that in humans is encoded by the HTR4 gene.

The serotonin 1A receptor is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered.

5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT).

5-Hydroxytryptamine (serotonin) receptor 5A, also known as HTR5A, is a protein that in humans is encoded by the HTR5A gene. Agonists and antagonists for 5-HT receptors, as well as serotonin uptake inhibitors, present promnesic (memory-promoting) and/or anti-amnesic effects under different conditions, and 5-HT receptors are also associated with neural changes.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Zacopride is a potent antagonist at the 5-HT3 receptor and an agonist at the 5-HT4 receptor. It has anxiolytic and nootropic effects in animal models, with the (R)-(+)-enantiomer being the more active form. It also has antiemetic and pro-respiratory effects, both reducing sleep apnea and reversing opioid-induced respiratory depression in animal studies. Early animal trials have also revealed that administration of zacopride can reduce preference for and consumption of ethanol.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and morphine or opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

GR-113808 is a drug which acts as a potent and selective 5-HT4 serotonin receptor antagonist. It is used in researching the roles of 5-HT4 receptors in various processes, and has been used to test some of the proposed therapeutic effects of selective 5-HT4 agonists, such as for instance blocking the nootropic effects of 5-HT4 agonists, and worsening the respiratory depression produced by opioid analgesic drugs, which appears to be partly 5-HT4 mediated and can be counteracted by certain 5-HT4 agonists.
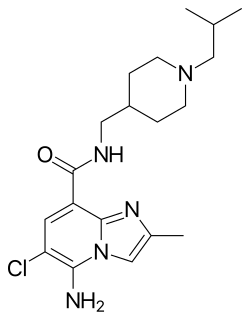
CJ-033466 is a drug which acts as a potent and selective 5-HT4 serotonin receptor partial agonist. In animal tests it stimulated gastrointestinal motility with 30 times the potency of cisapride, and with lower affinity for the hERG channel.

















