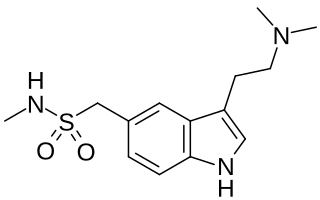
Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects generally occur within three hours.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Triptans are a family of tryptamine-based drugs used as abortive medication in the treatment of migraines and cluster headaches. This drug class was first commercially introduced in the 1990s. While effective at treating individual headaches, they do not provide preventive treatment and are not considered a cure. They are not effective for the treatment of tension–type headache, except in persons who also experience migraines. Triptans do not relieve other kinds of pain.

Dihydroergotamine (DHE), sold under the brand names D.H.E. 45 and Migranal among others, is an ergot alkaloid used to treat migraines. It is a derivative of ergotamine. It is administered as a nasal spray or injection and has an efficacy similar to that of sumatriptan. Nausea is a common side effect.
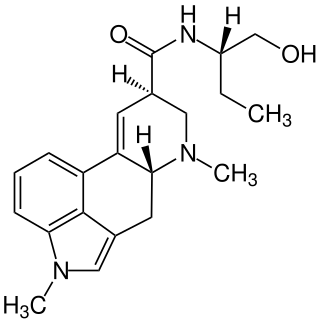
Methysergide, sold under the brand names Deseril and Sansert, is a monoaminergic medication of the ergoline and lysergamide groups which is used in the prophylaxis and treatment of migraine and cluster headaches. It has been withdrawn from the market in the United States and Canada due to adverse effects. It is taken by mouth.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.
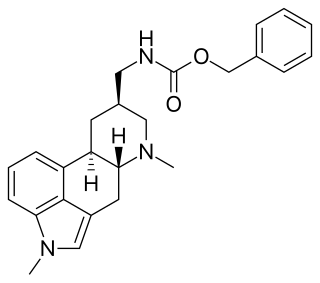
Metergoline, also known as methergoline and sold under the brand names Contralac (veterinary) and Liserdol (clinical), is a monoaminergic medication of the ergoline group which is used as a prolactin inhibitor in the treatment of hyperprolactinemia and to suppress lactation.

5-hydroxytryptamine receptor 1B also known as the 5-HT1B receptor is a protein that in humans is encoded by the HTR1B gene. The 5-HT1B receptor is a 5-HT receptor subtype.

5-hydroxytryptamine (serotonin) receptor 1D, also known as HTR1D, is a 5-HT receptor, but also denotes the human gene encoding it. 5-HT1D acts on the central nervous system, and affects locomotion and anxiety. It also induces vasoconstriction in the brain.

5-hydroxytryptamine (serotonin) receptor 1F, also known as HTR1F is a 5-HT1 receptor protein and also denotes the human gene encoding it.

LY-334370 is a selective 5-HT1F receptor agonist which was under development by Eli Lilly and Company for the treatment of migraine headaches. The drug showed efficacy in a phase II clinical trial but further development was halted due to toxicity detected in animals.
TCB-2 is a hallucinogen discovered in 2006 by Thomas McLean working in the lab of David Nichols at Purdue University. It is a conformationally-restricted derivative of the phenethylamine 2C-B, also a hallucinogen, and acts as a potent agonist for the 5-HT2A and 5-HT2C receptors with a Ki of 0.26 nM at the human 5-HT2A receptor. In drug-substitution experiments in rats, TCB-2 was found to be of similar potency to both LSD and Bromo-DragonFLY, ranking it among the most potent phenethylamine hallucinogens yet discovered. This high potency and selectivity has made TCB-2 useful for distinguishing 5-HT2A mediated responses from those produced by other similar receptors. TCB-2 has similar but not identical effects in animals to related phenethylamine hallucinogens such as DOI, and has been used for studying how the function of the 5-HT2A receptor differs from that of other serotonin receptors in a number of animal models, such as studies of cocaine addiction and neuropathic pain.

5-Carboxamidotryptamine (5-CT) is a tryptamine derivative closely related to the neurotransmitter serotonin.
Triptans is a word commonly used for a class of anti-migraine drugs that are selective 5-hydroxytryptamine/serotonin1B/1D (5-HT1B/1D) agonists. Migraine is a complex disease which affects about 15% of the population and can be highly disabling. Triptans have advantages over ergotamine and dihydroergotamine, such as selective pharmacology, well established safety record and evidence-based prescribing instructions. Triptans are therefore often preferred treatment in migraine.

CP-94253 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 25x and 40x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It has a range of behavioral effects, based on animal testing. The effects include the following: promoting wakefulness by increasing dopamine release in the brain; reducing food intake and promoting satiety; enhancing the reinforcing effects of cocaine; and possible antidepressant effects. A recent study found that "Regardless of sex, CP94253 decreased cocaine intake after abstinence and during resumption of SA [self-administration] and decreased cue reactivity" suggesting that agonism of the inhibitory 5-HT2B receptors may diminish the cognitive reward of cocaine usage and increased use of the drug without a period of abstinence may be a product of test subjects trying to achieve a previously rewarding experience through larger dosages of cocaine.
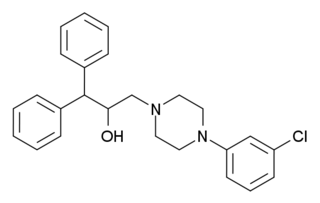
BRL-15,572 is a drug which acts as a selective antagonist for the serotonin receptor subtype 5-HT1D, with around 60x selectivity over other related receptors. The 5-HT1D receptor has a very similar pharmacology to the closely related 5-HT1B receptor, and most older ligands for these receptors bind to both subtypes with approximately equal affinity, so development of compounds such as BRL-15572 which are able to selectively block the 5-HT1D subtype while leaving 5-HT1B unaffected, have been a significant advance which has helped scientists in researching the function of these serotonin receptor subtypes. One function of the 5-HT1D receptor this research has revealed is its role in modulating release of the neurotransmitter glutamate in the brain, as well as functions in regulation of cerebral blood pressure which are important in the pathogenesis of migraine headaches.

CGS-12066A is a drug which acts as a potent and selective agonist for the 5-HT1B receptor with lower affinity for the three 5-HT2 receptor subtypes. It is used for studying the role of the 5-HT1B receptor in various processes including perception of pain and the sleep-wake cycle.

CP-93129 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 150x and 200x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It is used in the study of 5-HT1B receptors in the brain, particularly their role in modulating the release of other neurotransmitters.
The trigeminovascular system (TVS) refers to neurons and their axonal projections within the trigeminal nerve that project to the cranial meninges and meningeal blood vessels residing on the brain's surface. The term, introduced in 1983 denotes also the neuropeptides contained within axons that are released into the meninges to target vessels and surrounding cells.

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.
















