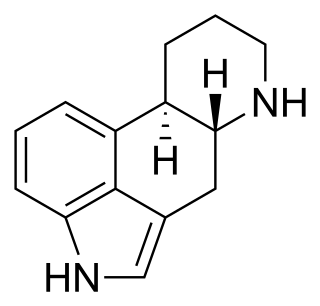
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

5-Fluoro-α-methyltryptamine, also known as PAL-544, is a putative stimulant, entactogen, and psychedelic tryptamine derivative related to α-methyltryptamine (αMT). It has been found to act as a well-balanced serotonin-norepinephrine-dopamine releasing agent, a 5-HT2A receptor agonist, and a potent and specific MAO-A inhibitor. which suggests that 5-fluoro-αMT could be an active psychedelic in humans, although it is not known to have been tested in humans and could be dangerous due to its strong inhibition of MAO-A.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to have been discovered.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.
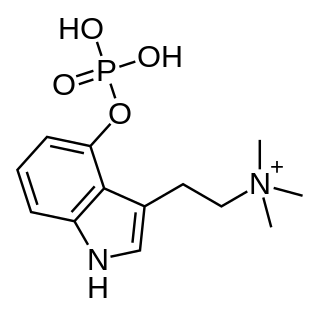
Aeruginascin, also known as 4-phosphoryloxy-N,N,N-trimethyltryptamine (4-PO-TMT), is an indoleamine derivative which occurs naturally within the mushrooms Inocybe aeruginascens, Pholiotina cyanopus, and Psilocybe cubensis.
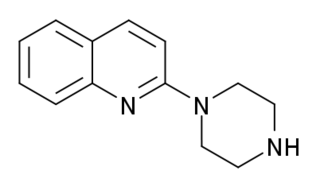
Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use.
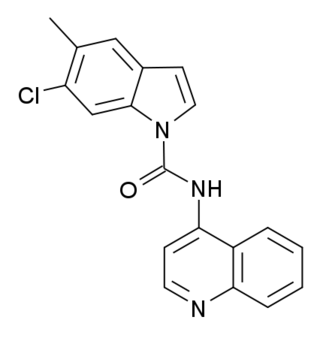
SB-215505 is a drug which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with good selectivity over the related 5-HT2A and 5-HT2C receptors. It is used in scientific research into the function of the 5-HT2 family of receptors, especially to study the role of 5-HT2B receptors in the heart, and to distinguish 5-HT2B-mediated responses from those produced by 5-HT2A or 5-HT2C.

7-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with reasonable selectivity for the D3 receptor subtype, and low affinity for serotonin receptors, unlike its structural isomer 8-OH-DPAT. 7-OH-DPAT is self-administered in several animal models, and is used to study its addiction effects to cocaine.
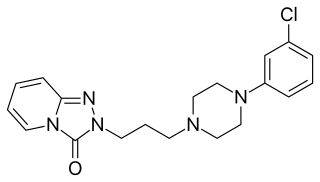
Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor.

CSP-2503 is a potent and selective 5-HT1A receptor agonist, 5-HT2A receptor antagonist, and 5-HT3 receptor antagonist of the naphthylpiperazine class. First synthesized in 2003, it was designed based on computational models and QSAR studies. In rat studies, CSP-2503 has demonstrated anxiolytic effects, and thus has been suggested as a treatment for anxiety in humans with a multimodal mechanism of action.
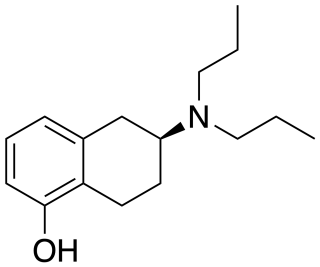
5-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with selectivity for the D2 receptor and D3 receptor subtypes. Only the (S)-enantiomer is active as an agonist, with the (R)-enantiomer being a weak antagonist at D2 receptors. Radiolabelled 11C-5-OH-DPAT is used as an agonist radioligand for mapping the distribution and function of D2 and D3 receptors in the brain, and the drug is also being studied in the treatment of Parkinson's disease.

The head-twitch response (HTR) is a rapid side-to-side head movement that occurs in mice and rats after the serotonin 5-HT2A receptor is activated. The prefrontal cortex may be the neuroanatomical locus mediating the HTR. Many serotonergic hallucinogens, including lysergic acid diethylamide (LSD), induce the head-twitch response, and so the HTR is used as a behavioral model of hallucinogen effects. However while there is generally a good correlation between compounds that induce head twitch in mice and compounds that are hallucinogenic in humans, it is unclear whether the head twitch response is primarily caused by 5-HT2A receptors, 5-HT2C receptors or both, though recent evidence shows that the HTR is mediated by the 5-HT2A receptor and modulated by the 5-HT2C receptor. Also, the effect can be non-specific, with head twitch responses also produced by some drugs that do not act through 5-HT2 receptors, such as phencyclidine, yohimbine, atropine and cannabinoid receptor antagonists. As well, compounds such as 5-HTP, fenfluramine, 1-Methylpsilocin, Ergometrine, and 3,4-di-methoxyphenethylamine (DMPEA) can also produce head twitch and do stimulate serotonin receptors, but are not hallucinogenic in humans. This means that while the head twitch response can be a useful indicator as to whether a compound is likely to display hallucinogenic activity in humans, the induction of a head twitch response does not necessarily mean that a compound will be hallucinogenic, and caution should be exercised when interpreting such results.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

1-Methylpsilocin (developmental code names CMY, CMY-16) is a tryptamine derivative developed by Sandoz which acts as a selective agonist of the serotonin 5-HT2C receptor (IC50Tooltip half-maximal inhibitory concentration of 12 nM, vs. 633 nM at 5-HT2A), and an inverse agonist at 5-HT2B (Ki of 38 nM). While 1-methylpsilocin does have higher affinity for 5-HT2C than 5-HT2A, it does produce a head-twitch response in mice that is dependent on 5-HT2A, so it is not entirely free of effects on 5-HT2Ain vivo. In contrast to psilocin, 1-methylpsilocin did not activate 5-HT1A receptors in mice.




















