
Quinpirole is a psychoactive drug and research chemical which acts as a selective D2 and D3 receptor agonist. It is used in scientific research. Quinpirole has been shown to increase locomotion and sniffing behavior in mice treated with it. At least one study has found that quinpirole induces compulsive behavior symptomatic of obsessive compulsive disorder in rats. Another study in rats show that quinpirole produces significant THC-like effects when metabolic degradation of anandamide is inhibited, supporting the hypothesis that these effects of quinpirole are mediated by cannabinoid CB1 receptors. Quinpirole may also reduce relapse in adolescent rat models of cocaine addiction.
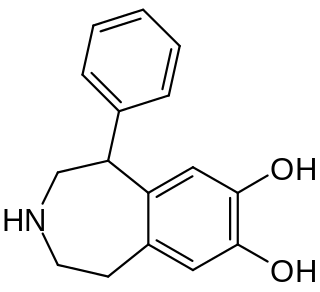
SKF-38,393 is a synthetic compound of the benzazepine chemical class which acts as a selective D1/D5 receptor partial agonist. It has stimulant and anorectic effects.
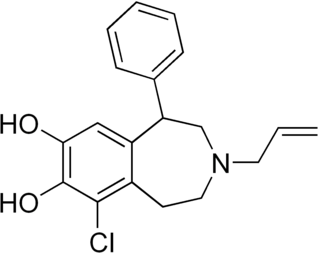
SKF-82,958 is a synthetic compound of the benzazepine class that acts as a D1/D5 receptor full agonist. SKF-82,958 and similar D1-like-selective full agonists like SKF-81,297 and 6-Br-APB produce characteristic anorectic effects, hyperactivity and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine. SKF-82,958 was also subsequently found to act as an agonist of ERα with negligible activity at ERβ, making it a subtype-selective estrogen.

Dopamine receptor D2, also known as D2R, is a protein that, in humans, is encoded by the DRD2 gene. After work from Paul Greengard's lab had suggested that dopamine receptors were the site of action of antipsychotic drugs, several groups, including those of Solomon Snyder and Philip Seeman used a radiolabeled antipsychotic drug to identify what is now known as the dopamine D2 receptor. The dopamine D2 receptor is the main receptor for most antipsychotic drugs. The structure of DRD2 in complex with the atypical antipsychotic risperidone has been determined.

The serotonin 1A receptor is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

N-n-Propylnorapomorphine (NPA) is an aporphine derivative dopamine agonist closely related to apomorphine. In rodents it has been shown to produce hyperactivity, stereotypy, hypothermia, antinociception, and penile erection, among other effects. Notably, its effects on locomotion are biphasic, with low doses producing inhibition and catalepsy and high doses resulting in enhancement of activity. This is likely due to preferential activation of D2/D3 autoreceptors versus postsynaptic receptors, the latter of which overcomes the former to increase postsynaptic dopaminergic signaling only with high doses.
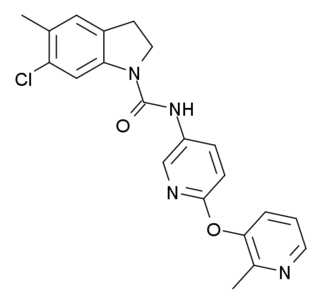
SB-242084 is a psychoactive drug and research chemical which acts as a selective antagonist for the 5HT2C receptor. It has anxiolytic effects, and enhances dopamine signalling in the limbic system, as well as having complex effects on the dopamine release produced by cocaine, increasing it in some brain regions but reducing it in others. It has been shown to increase the effectiveness of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants, and may also reduce their side effects. In animal studies, SB-242084 produced stimulant-type activity and reinforcing effects, somewhat similar to but much weaker than cocaine or amphetamines.

Chloroethylnorapomorphine is a chemical once thought to be an irreversible dopamine D2 receptor antagonist; however, it was later proved to be reversible.

BIIE-0246 is a drug used in scientific research which acts as a potent and selective antagonist for the Neuropeptide Y receptor Y2. It was one of the first non-peptide Y2-selective antagonists developed, and remains among the most widely used tools for studying this receptor. It has been used to demonstrate a role for the Y2 subtype as a presynaptic autoreceptor limiting further neuropeptide Y release, as well as modulating dopamine and acetylcholine release. It has also been shown to produce several behavioural effects in animals, including reducing alcohol consumption in addicted rats and anxiolytic effects, although while selective Y2 agonists are expected to be useful as anorectics, BIIE-0246 did not appear to increase appetite when administered alone.
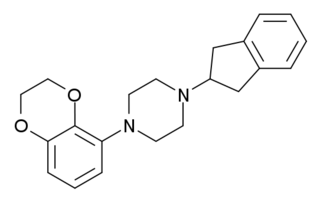
S-15535 is a phenylpiperazine drug which is a potent and highly selective 5-HT1A receptor ligand that acts as an agonist and antagonist at the presynaptic and postsynaptic 5-HT1A receptors, respectively. It has anxiolytic properties.

6-Br-APB is a synthetic compound that acts as a selective D1 agonist, with the (R)-enantiomer being a potent full agonist, while the (S) enantiomer retains its D1 selectivity but is a weak partial agonist. (R)-6-Br-APB and similar D1-selective full agonists like SKF-81,297 and SKF-82,958 produce characteristic anorectic effects, stereotyped behaviour and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine.
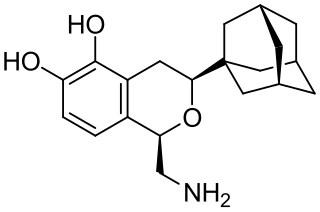
A-77636 is a synthetic drug which acts as a selective D1 receptor full agonist. It has nootropic, anorectic, rewarding and antiparkinsonian effects in animal studies, but its high potency and long duration of action causes D1 receptor downregulation and tachyphylaxis, and unlike other D1 full agonists such as SKF-82,958, it does not produce place preference in animals. A-77636 partially substituted for cocaine in animal studies, and has been suggested for use as a possible substitute drug in treating addiction, but it is better known for its use in studying the role of D1 receptors in the brain.

A-68930 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. It is orally active and has antidepressant and anorectic effects in animals, producing wakefulness and tachycardia, but without stimulant effects, instead producing sedation. The difference in effects between A-68930 and other D1 agonists such as SKF-82958 may be due to their differing effects on the related D5 receptor.

7-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with reasonable selectivity for the D3 receptor subtype, and low affinity for serotonin receptors, unlike its structural isomer 8-OH-DPAT. 7-OH-DPAT is self-administered in several animal models, and is used to study addiction to cocaine.

8-OH-PBZI is a drug used in scientific research which acts as a potent and selective agonist for the dopamine D3 receptor.
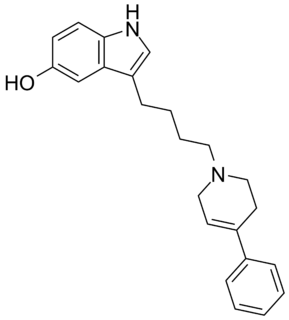
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.
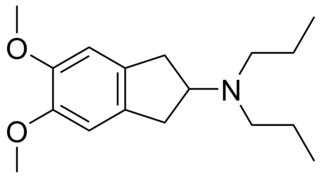
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

OSU-6162 (PNU-96391) is a compound which acts as a partial agonist at both dopamine D2 receptors and 5-HT2A receptors. It acts as a dopamine stabilizer in a similar manner to the closely related drug pridopidine, and has antipsychotic, anti-addictive and anti-Parkinsonian effects in animal studies. Both enantiomers show similar activity but with different ratios of effects, with the (S) enantiomer (–)-OSU-6162 that is more commonly used in research, having higher binding affinity to D2 but is a weaker partial agonist at 5-HT2A, while the (R) enantiomer (+)-OSU-6162 has higher efficacy at 5-HT2A but lower D2 affinity.

PNU-91356A (U-91356) is a drug used in scientific research which acts as a potent and reasonably selective agonist of the dopamine receptor D2, with lower affinity for the related D3 and D4 subtypes and the 5-HT1A receptor.




















