
A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.

Sympathomimetic drugs are stimulant compounds which mimic the effects of endogenous agonists of the sympathetic nervous system. Examples of sympathomimetic effects include increases in heart rate, force of cardiac contraction, and blood pressure. The primary endogenous agonists of the sympathetic nervous system are the catecholamines, which function as both neurotransmitters and hormones. Sympathomimetic drugs are used to treat cardiac arrest and low blood pressure, or even delay premature labor, among other things.
An adrenergic agonist is a drug that stimulates a response from the adrenergic receptors. The five main categories of adrenergic receptors are: α1, α2, β1, β2, and β3, although there are more subtypes, and agonists vary in specificity between these receptors, and may be classified respectively. However, there are also other mechanisms of adrenergic agonism. Epinephrine and norepinephrine are endogenous and broad-spectrum. More selective agonists are more useful in pharmacology.

Synephrine, or, more specifically, p-synephrine, is an alkaloid, occurring naturally in some plants and animals, and also in approved drugs products as its m-substituted analog known as neo-synephrine. p-Synephrine and m-synephrine are known for their longer acting adrenergic effects compared to epinephrine and norepinephrine. This substance is present at very low concentrations in common foodstuffs such as orange juice and other orange products, both of the "sweet" and "bitter" variety. The preparations used in traditional Chinese medicine (TCM), also known as Zhi Shi (枳实), are the immature and dried whole oranges from Citrus aurantium. Extracts of the same material or purified synephrine are also marketed in the US, sometimes in combination with caffeine, as a weight-loss-promoting dietary supplement for oral consumption. While the traditional preparations have been in use for millennia as a component of TCM-formulas, synephrine itself is not an approved over the counter drug. As a pharmaceutical, m-synephrine (phenylephrine) is still used as a sympathomimetic, mostly by injection for the treatment of emergencies such as shock, and rarely orally for the treatment of bronchial problems associated with asthma and hay-fever.

Isoprenaline, also known as isoproterenol and sold under the brand name Isuprel among others, is a sympathomimetic medication which is used in the treatment of acute bradycardia, heart block, and rarely for asthma, among other indications. It is used by injection into a vein, muscle, fat, or the heart, by inhalation, and in the past under the tongue or into the rectum.

Dobutamine is a medication used in the treatment of cardiogenic shock and severe heart failure. It may also be used in certain types of cardiac stress tests. It is given by IV only, as an injection into a vein or intraosseous as a continuous infusion. The amount of medication needs to be adjusted to the desired effect. Onset of effects is generally seen within 2 minutes. It has a half-life of two minutes. This drug is generally only administered short term, although it may be used for longer periods to relieve symptoms of heart failure in patients awaiting heart transplantation.
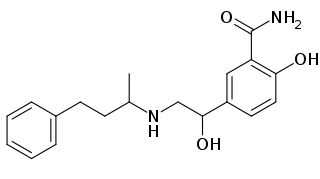
Labetalol is a medication used to treat high blood pressure and in long term management of angina. This includes essential hypertension, hypertensive emergencies, and hypertension of pregnancy. In essential hypertension it is generally less preferred than a number of other blood pressure medications. It can be given by mouth or by injection into a vein.

Hordenine is an alkaloid of the phenethylamine class that occurs naturally in a variety of plants, taking its name from one of the most common, barley. Chemically, hordenine is the N-methyl derivative of N-methyltyramine, and the N,N-dimethyl derivative of the well-known biogenic amine tyramine, from which it is biosynthetically derived and with which it shares some pharmacological properties. As of September 2012, hordenine is widely sold as an ingredient of nutritional supplements, with the claims that it is a stimulant of the central nervous system, and has the ability to promote weight loss by enhancing metabolism. In experimental animals, given sufficiently large doses parenterally, hordenine does produce an increase in blood pressure, as well as other disturbances of the cardiovascular, respiratory, and nervous systems. These effects are generally not reproduced by oral administration of the drug in test animals, and virtually no scientific reports of the effects of hordenine in human beings have been published.

N-Methylphenethylamine (NMPEA) is a naturally occurring trace amine neuromodulator in humans that is derived from the trace amine, phenethylamine (PEA). It has been detected in human urine and is produced by phenylethanolamine N-methyltransferase with phenethylamine as a substrate, which significantly increases PEA's effects. PEA breaks down into phenylacetaldehyde which is further broken down into phenylacetic acid by monoamine oxidase. When this is inhibited by monoamine oxidase inhibitors, it allows more of the PEA to be metabolized into nymphetamine (NMPEA) and not wasted on the weaker inactive metabolites.
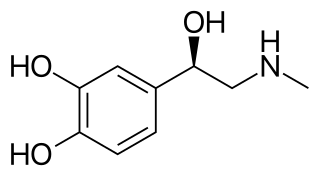
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions. It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata. It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node, pupil dilation response, and blood sugar level. It does this by binding to alpha and beta receptors. It is found in many animals, including humans, and some single-celled organisms. It has also been isolated from the plant Scoparia dulcis found in Northern Vietnam.

Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum, Annona squamosa, and Nelumbo nucifera.
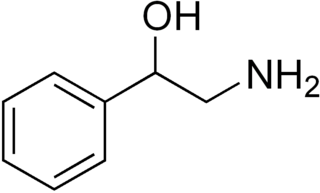
Phenylethanolamine, or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name Apophedrin, has been used as a drug to produce topical vasoconstriction.

N-Methyltyramine (NMT), also known as 4-hydroxy-N-methylphenethylamine, is a human trace amine and natural phenethylamine alkaloid found in a variety of plants. As the name implies, it is the N-methyl analog of tyramine, which is a well-known biogenic trace amine with which NMT shares many pharmacological properties. Biosynthetically, NMT is produced by the N-methylation of tyramine via the action of the enzyme phenylethanolamine N-methyltransferase in humans and tyramine N-methyltransferase in plants.

Candicine is a naturally occurring organic compound that is a quaternary ammonium salt with a phenethylamine skeleton. It is the N,N,N-trimethyl derivative of the well-known biogenic amine tyramine, and, being a natural product with a positively charged nitrogen atom in its molecular structure, it is classed as an alkaloid. Although it is found in a variety of plants, including barley, its properties have not been extensively studied with modern techniques. Candicine is toxic after parenteral administration, producing symptoms of neuromuscular blockade; further details are given in the "Pharmacology" section below.

N,N-Dimethyldopamine (DMDA) is an organic compound belonging to the phenethylamine family. It is related structurally to the alkaloid epinine (N-methyldopamine) and to the major neurotransmitter dopamine (of which it is the N,N-dimethylated analog). Because of its structural relationship to dopamine, DMDA has been the subject of a number of pharmacological investigations. DMDA has been detected in Acacia rigidula.

Halostachine is a natural product, an alkaloid first isolated from the Asian shrub Halostachys caspica, and structurally a β-hydroxy-phenethylamine related to its better-known "parent" biogenic amine, phenylethanolamine, to the adrenergic drug synephrine, and to the alkaloid ephedrine. The pharmacological properties of halostachine have some similarity to those of these structurally-related compounds, and Halostachys caspica extracts have been included as a constituent of certain OTC dietary supplements, but halostachine has never been developed as a prescription drug. Although it is found in nature as a single stereoisomer, halostachine is more commonly available as a synthetic product in the form of its racemate. In appearance it is a colorless solid.
The catecholamines are a group of neurotransmitters composed of the endogenous substances dopamine, noradrenaline (norepinephrine), and adrenaline (epinephrine), as well as numerous artificially synthesized compounds such as isoprenaline - an anti-bradycardiac medication. Their investigation constitutes a major chapter in the history of physiology, biochemistry, and pharmacology. Adrenaline was the first hormone extracted from an endocrine gland and obtained in pure form, before the word hormone was coined. Adrenaline was also the first hormone whose structure and biosynthesis was discovered. Second to acetylcholine, adrenaline and noradrenaline were some of the first neurotransmitters discovered, and the first intercellular biochemical signals to be found in intracellular vesicles. The β-adrenoceptor gene was the first G protein-coupled receptor to be cloned.
β2-adrenoceptor agonists are a group of drugs that act selectively on β2-receptors in the lungs causing bronchodilation. β2-agonists are used to treat asthma and COPD, diseases that cause obstruction in the airways. Prior to their discovery, the non-selective beta-agonist isoprenaline was used. The aim of the drug development through the years has been to minimise side effects, achieve selectivity and longer duration of action. The mechanism of action is well understood and has facilitated the development. The structure of the binding site and the nature of the binding is also well known, as is the structure activity relationship.
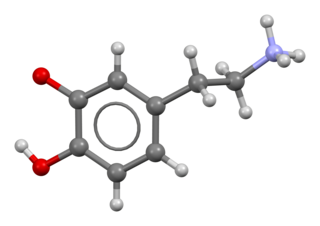
Dopamine, sold under the brand name Intropin among others, is a medication most commonly used in the treatment of very low blood pressure, a slow heart rate that is causing symptoms, and, if epinephrine is not available, cardiac arrest. In newborn babies it continues to be the preferred treatment for very low blood pressure. In children epinephrine or norepinephrine is generally preferred while in adults norepinephrine is generally preferred for very low blood pressure. It is given intravenously or intraosseously as a continuous infusion. Effects typically begin within five minutes. Doses are then increased to effect.


















