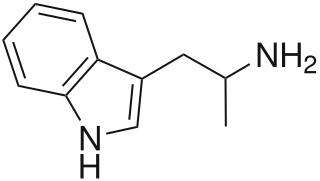
α-Methyltryptamine is a psychedelic, stimulant, and entactogen drug of the tryptamine family. It was originally developed as an antidepressant at Upjohn in the 1960s, and was used briefly as an antidepressant in the Soviet Union under the brand name Indopan or Indopane before being discontinued.

Tryptamine is an indolamine metabolite of the essential amino acid tryptophan. The chemical structure is defined by an indole—a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon. The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others.
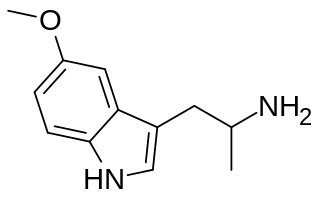
5-MeO-αMT, or 5-methoxy-α-methyltryptamine, also known as α,O-dimethylserotonin (Alpha-O), is a serotonergic psychedelic of the tryptamine family. It is a derivative of α-methyltryptamine (αMT) and an analogue of 5-MeO-DMT.
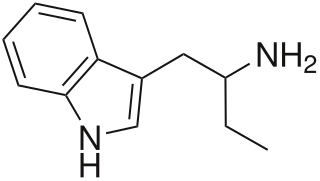
α-Ethyltryptamine, also known as etryptamine, is an entactogen and stimulant drug of the tryptamine family. It was originally developed and marketed as an antidepressant under the brand name Monase by Upjohn in the 1960s before being withdrawn due to toxicity.

N-Methyltryptamine (NMT), also known as monomethyltryptamine, is a chemical compound of the tryptamine family and a naturally occurring compound found in the human body and certain plants.
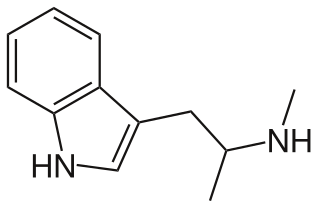
α,N-Dimethyltryptamine (α,N-DMT; developmental code names SK&F-7024, Ro 3-1715), also known as N-methyl-α-methyltryptamine (N-methyl-αMT), is a lesser-known substituted tryptamine and psychoactive drug. It is the α,N-dimethyl positional isomer of N,N-dimethyltryptamine (N,N-DMT).

5-Fluoro-α-methyltryptamine, also known as PAL-212 or PAL-544, is a putative stimulant, entactogen, and psychedelic tryptamine derivative related to α-methyltryptamine (αMT).
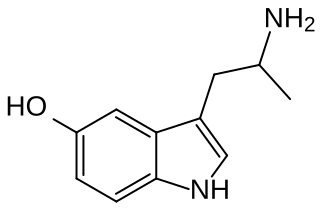
α-Methylserotonin (αMS), also known as α-methyl-5-hydroxytryptamine (α-methyl-5-HT) or 5-hydroxy-α-methyltryptamine (5-HO-αMT), is a tryptamine derivative closely related to the neurotransmitter serotonin (5-HT). It acts as a non-selective serotonin receptor agonist and has been used extensively in scientific research to study the function of the serotonin system.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; monoamine releasing agents can induce the release of one or more of these neurotransmitters.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons, including dopamine and norepinephrine neurons.

A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain.
A serotonin–norepinephrine–dopamine releasing agent (SNDRA), also known as a triple releasing agent (TRA), is a type of drug which induces the release of serotonin, norepinephrine/epinephrine, and dopamine in the brain and body. SNDRAs produce euphoriant, entactogen, and psychostimulant effects, and are almost exclusively encountered as recreational drugs.
A serotonin–dopamine releasing agent (SDRA) is a type of drug which induces the release of serotonin and dopamine in the body and/or brain.
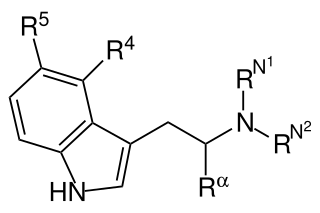
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.
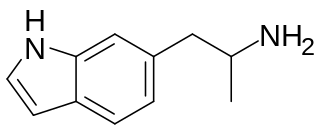
6-(2-Aminopropyl)indole is an indole derivative which was first identified being sold on the designer drug market by a laboratory in the Czechia in July 2016.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.
5-MAPB, also known as 5-(N-methyl-2-aminopropyl)benzofuran, is an entactogen and designer drug of the amphetamine family that is similar to MDMA in its structure and effects.

5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative related to α-methyltryptamine (αMT) and one of only a few known specific serotonin-dopamine releasing agents (SDRAs). It has been investigated in animals as a potential treatment for cocaine dependence. The EC50 values of 5-chloro-αMT in evoking the in vitro release of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) in rat synaptosomes were reported as 16 nM, 54 nM, and 3434 nM, with an NE/DA ratio of 63.6 and a DA/5-HT ratio of 3.38, indicating that it is a highly specific and well-balanced SDRA. However, 5-chloro-αMT has also been found to act as a potent full agonist of the 5-HT2A receptor, with an EC50 value of 6.27 nM and an efficacy of 105%. It is likely to act as a potent agonist of other serotonin receptors as well.

(R)-3,4-Methylenedioxy-N-methylamphetamine ((R)-MDMA), also known as (R)-midomafetamine or as levo-MDMA, is the (R)- or levorotatory (l-) enantiomer of 3,4-methylenedioxy-N-methylamphetamine (MDMA; midomafetamine; "ecstasy"), a racemic mixture of (R)-MDMA and (S)-MDMA. Like MDMA, (R)-MDMA is an entactogen or empathogen. It is taken by mouth.

BK-NM-AMT, or βk-NM-αMT, also known as β-keto-N-methyl-αMT or as α,N-dimethyl-β-ketotryptamine, is a serotonin–dopamine releasing agent (SDRA) and putative entactogen of the tryptamine and α-alkyltryptamine families. Along with certain other tryptamines, such as α-ethyltryptamine (αET), 5-chloro-αMT and 5-fluoro-αET, it is one of the few SDRAs known.
















