Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), and obsessive–compulsive disorder (OCD). SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs, however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

Nomifensine, sold under the brand names Merital and Alival, is a norepinephrine–dopamine reuptake inhibitor (NDRI), i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters. This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Indatraline hydrochloride is an antidepressive agent and non-selective monoamine transporter inhibitor that blocks the reuptake of dopamine, norepinephrine, and serotonin with similar efficacy to cocaine. This compound may be used to treat cocaine addictions as its effects have a slower onset and a longer duration than those of cocaine. Lu 19-005 has been shown to block the action of methamphetamine and MDMA in laboratory experiments.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Diclofensine (Ro 8-4650) was developed by Hoffmann-La Roche in the 1970s in the search for a new antidepressant. It was found that the (S)-isomer was responsible for activity. Diclofensine is a stimulant drug which acts as a triple monoamine reuptake inhibitor, primarily inhibiting the reuptake of dopamine and norepinephrine, with affinities (Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT (dopamine, norepinephrine and serotonin transporters), respectively. It was found to be an effective antidepressant in human trials, with relatively few side effects, but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential.
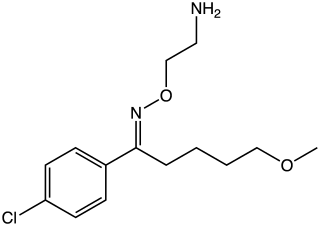
Clovoxamine (INN) is a drug that was discovered in the 1970s and was subsequently investigated as an antidepressant and anxiolytic agent but was never marketed. It acts as a serotonin-norepinephrine reuptake inhibitor (SNRI), with little affinity for the muscarinic acetylcholine, histamine, adrenergic, and serotonin receptors. The compound is structurally related to fluvoxamine.

Oxaprotiline, also known as hydroxymaprotiline, is a norepinephrine reuptake inhibitor belonging to the tetracyclic antidepressant (TeCA) family and is related to maprotiline. Though investigated as an antidepressant, it was never marketed.

Ciclazindol (WY-23409) is an antidepressant and anorectic drug of the tetracyclic chemical class that was developed in the mid to late 1970s, but was never marketed. It acts as a norepinephrine reuptake inhibitor, and to a lesser extent as a dopamine reuptake inhibitor. Ciclazindol has no effects on the SERT, 5-HT receptors, mACh receptors, or α-adrenergic receptors, and has only weak affinity for the H1 receptor. As suggested by its local anesthetic properties, ciclazindol may also inhibit sodium channels. It is known to block potassium channels as well.

A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain.
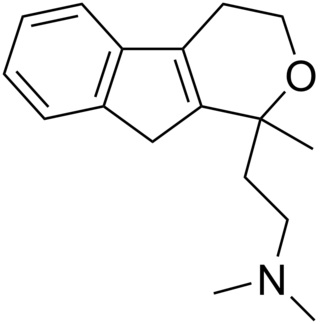
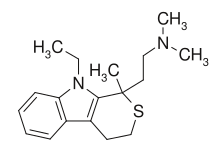
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.

Talsupram is a selective norepinephrine reuptake inhibitor (NRI) which was investigated as an antidepressant in the 1960s and 1970s but was never marketed. Along with talopram, it is structurally related to the selective serotonin reuptake inhibitor (SSRI) citalopram.
EXP-561 is an investigational drug that acts as an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. It was developed in the 1960s by Du Pont and was suggested as a potential antidepressant but failed in trials and was never marketed.

Pridefine (AHR-1,118) is a drug which was investigated as an antidepressant in the late 1970s and early 1980s, but was never marketed. It acts as a balanced reuptake inhibitor of serotonin, dopamine, and norepinephrine, and also has some weak releasing activity.
Selective serotonin reuptake inhibitors, or serotonin-specific re-uptake inhibitor (SSRIs), are a class of chemical compounds that have application as antidepressants and in the treatment of depression and other psychiatric disorders. SSRIs are therapeutically useful in the treatment of panic disorder (PD), posttraumatic stress disorder (PTSD), social anxiety disorder, obsessive-compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD), and anorexia. There is also clinical evidence of the value of SSRIs in the treatment of the symptoms of schizophrenia and their ability to prevent cardiovascular diseases.