 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H23NO |
| Molar mass | 281.399 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
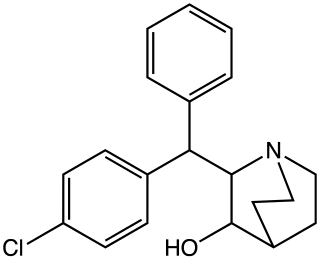
SCH-5472. [1] is a stimulant drug [2] developed by Schering-Plough in the 1950s. [1]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H23NO |
| Molar mass | 281.399 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
SCH-5472. [1] is a stimulant drug [2] developed by Schering-Plough in the 1950s. [1]

Potassium amide was made from the reaction of potassium metal with liquid ammonia. The coupling between the carbanion created from diphenylmethane [101-81-5] (1) and ethyl 2-furoate [614-99-3] [1335-40-6] (2) gives 2-furyl benzhydryl ketone, CID:63950182 (3). This was reacted with concentrated liquid ammonia in methanol solvent in an autoclave. The product of this step was 2-benzhydrylpyridin-3-ol, CID:125491889 (4). Reduction of the pyridine gave 2-(diphenylmethyl)piperidin-3-ol, CID:209477 (5).

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Ammonia is an inorganic compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Potassium is the chemical element with the symbol K and atomic number 19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge. In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in seawater, and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

Hydroxylamine is an inorganic compound with the formula NH2OH. The material is a white crystalline, hygroscopic compound. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. The oxidation of NH3 to hydroxylamine is a step in biological nitrification.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.

Calcium nitrate, also called Norgessalpeter or Norwegian salpeter, is an inorganic compound with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the air to give the tetrahydrate. Both anhydrous and hydrated forms are colourless salts. Calcium nitrate is mainly used as a component in fertilizers, but it has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of double salts are known including calcium ammonium nitrate decahydrate (NH4NO3·5Ca(NO3)2·10H2O) and calcium potassium nitrate (Ca(NO3)2·4KNO3).

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
A solvated electron is a free electron in a solution, and is the smallest possible anion. Solvated electrons occur widely. Often, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but solvated electrons also occur in water and other solvents – in fact, in any solvent that mediates outer-sphere electron transfer. The solvated electron is responsible for a great deal of radiation chemistry.
Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. As a result, sodium usually forms ionic compounds involving the Na+ cation. Sodium is a reactive alkali metal and is much more stable in ionic compounds. It can also form intermetallic compounds and organosodium compounds. Sodium compounds are often soluble in water.
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent.

Metitepine, also known as methiothepin, is a drug described as a "psychotropic agent" of the tricyclic group which was never marketed. It acts as a non-selective antagonist of serotonin, dopamine, and adrenergic receptors and has antipsychotic properties.

Acetylenediol, or ethynediol, is a chemical substance with formula HO−C≡C−OH (an ynol). It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal (CHO)2 is well known.
AL-1095, is a centrally acting stimulant drug with comparable effects to amphetamine, developed by Bristol in the 1970s.

Difemetorex, also known as diphemethoxidine, is a stimulant drug of the piperidine class which was used as an appetite suppressant, but produced intolerable side effects such as insomnia which limited its clinical use. It was introduced in France by Ciba-Geigy in 1966 but is now no longer marketed.

Carfenazine (INN), or carphenazine (BAN), also known as carphenazine maleate (USAN), is an antipsychotic and tranquilizer of the phenothiazine group that was withdrawn from the market.
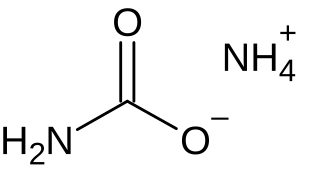
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.
In chemistry, ammonolysis (/am·mo·nol·y·sis/) is the process of splitting ammonia into . Ammonolysis reactions can be conducted with organic compounds to produce amines (molecules containing a nitrogen atom with a lone pair, :N), or with inorganic compounds to produce nitrides. This reaction is analogous to hydrolysis in which water molecules are split. Similar to water, liquid ammonia also undergoes auto-ionization, , where the rate constant is k = 1.9 × 10-38.

Potassium telluride is an inorganic compound with a chemical formula K2Te. It is formed from potassium and tellurium, making it a telluride. Potassium telluride is a white powder. Like rubidium telluride and caesium telluride, it can be used as an ultraviolet detector in space. Its crystal structure is similar to other tellurides, which have an anti-fluorite structure.