Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.

Meclofenoxate is a cholinergic nootropic used as a dietary supplement. It is an ester of dimethylethanolamine (DMAE) and 4-chlorophenoxyacetic acid (pCPA).
Isobutyl nitrite, C4H9NO2, is an alkyl nitrite, an ester of isobutanol and nitrous acid. Its chemical structure is (CH3)2CH-CH2-ONO.

Boldenone, is a naturally occurring anabolic–androgenic steroid (AAS) and the 1(2)-dehydrogenated analogue of testosterone. Boldenone itself has never been marketed; as a pharmaceutical drug, it is used as boldenone undecylenate, the undecylenate ester.
Parecoxib, sold under the brand name Dynastat among others, is a water-soluble and injectable prodrug of valdecoxib. Parecoxib is a COX2 selective inhibitor. It is injectable. It is approved in the European Union for short term perioperative pain control.

Levomethorphan (LVM) (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine.

Iproclozide is an irreversible and selective monoamine oxidase inhibitor (MAOI) of the hydrazine chemical class that was used as an antidepressant, but has since been discontinued. It has been known to cause fulminant hepatitis and there have been at least three reported fatalities due to administration of the drug.

Prolintane is a central nervous system (CNS) stimulant and norepinephrine–dopamine reuptake inhibitor (NDRI) developed in the 1950s. Being an amphetamine derivative, it is closely related in chemical structure to other drugs such as pyrovalerone, MDPV, and propylhexedrine, and has a similar mechanism of action. Many cases of prolintane abuse have been reported.

Etafedrine, sold under the brand name Nethaprin among others and also known as N-ethylephedrine, is a sympathomimetic agent used as a bronchodilator to treat asthma. It was previously commercially available as both the free base and as the hydrochloride salt from Sanofi-Aventis but is now no longer marketed.
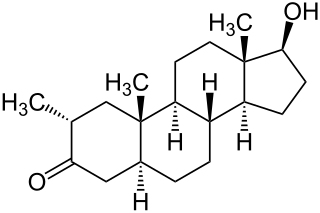
Drostanolone, or dromostanolone, is an anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was never marketed. An androgen ester prodrug of drostanolone, drostanolone propionate, was formerly used in the treatment of breast cancer in women under brand names such as Drolban, Masteril, and Masteron. This has also been used non-medically for physique- or performance-enhancing purposes.

Oxypertine, sold under the brand name Oxypertine among others, is an antipsychotic medication of the tryptamine and phenylpiperazine groups which was previously used in the treatment of schizophrenia but is no longer marketed. It was also evaluated for the treatment of anxiety.
Pheniprazine, formerly sold under the brand names Catron and Cavodil, is an irreversible and non-selective monoamine oxidase inhibitor (MAOI) of the hydrazine group that was used as an antidepressant to treat depression in the 1960s. It was also used in the treatment of angina pectoris and schizophrenia. Pheniprazine has been largely discontinued due to toxicity concerns such as jaundice, amblyopia, and optic neuritis.

Dixyrazine, also known as dixypazin (oxalate), sold under the brand names Ansiolene, Esocalm, Esucos, Metronal, and Roscal, is a typical antipsychotic of the phenothiazine group described as a neuroleptic and antihistamine. It was first introduced in Germany in 1969. It is used as a neuroleptic, anxiolytic, and antihistamine in doses between 12.5 and 75 mg a day.

Prothipendyl, also known as azapromazine or phrenotropin, is an anxiolytic, antiemetic, and antihistamine of the azaphenothiazine group which is marketed in Europe and is used to treat anxiety and agitation in psychotic syndromes. It differs from promazine only by the replacement of one carbon atom with a nitrogen atom in the tricyclic ring system. Prothipendyl is said to not possess antipsychotic effects, and in accordance, appears to be a weaker dopamine receptor antagonist than other phenothiazines.

Bolasterone, also known as 7α,17α-dimethyltestosterone, is a 17α-alkylated androgen/anabolic steroid (AAS) which is used in veterinary medicine. It has close structural similarity to testosterone, and like methyltestosterone has a methyl group at C17α in order to increase oral bioavailability. In addition, it is also 7α-methylated, similar to its 7β-methylated isomer calusterone. The medication has a low to moderate ratio of anabolic to androgenic activity, similar to that of fluoxymesterone.

Formebolone, also known as formyldienolone, as well as 2-formyl-11α-hydroxy-17α-methyl-δ1-testosterone, is an orally active anabolic-androgenic steroid (AAS) described as an anticatabolic and anabolic drug that is or has been marketed in Spain and Italy. As an AAS, it shows some anabolic activity, though it is inferior to testosterone in terms of potency, but is said to have virtually no androgenic activity. Formebolone counteracts the catabolic effects of potent glucocorticoids like dexamethasone phosphate. A close analogue, roxibolone, shows similar antiglucocorticoid activity to formebolone but, in contrast, is devoid of activity as an AAS.

Oxymesterone, also known as methandrostenediolone, as well as 4-hydroxy-17α-methyltestosterone or 17α-methylandrost-4-en-4,17β-diol-3-one, is an orally active anabolic-androgenic steroid (AAS). It was known by 1960.

Cyclarbamate, also known as cyclopentaphene, is a muscle relaxant and tranquilizer of the carbamate family which has been marketed by Cassenne in France since 1961.
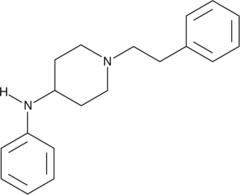
4-ANPP, also known as 4-anilino-N-phenethylpiperidine (4-ANPP), 4-aminophenyl-1-phenethylpiperidine, or despropionyl fentanyl, is a direct precursor to fentanyl and acetylfentanyl. It is commonly found as a contaminant in samples of drugs containing fentanyl, which may include samples represented by the supplier as heroin or other opioids. It is not psychoactive and is present only as a result of improper chemical purification.

Hydroxyphenamate or oxyfenamate is a sedative and anxiolytic drug of the carbamate class which is no longer marketed in the US. Like other carbamate sedatives, it is chemically related to meprobamate (Miltown). It was introduced to the US market in 1961. The dosage for adults is 200 mg 3 to 4 times daily.