
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.

Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.
A structural analog, also known as a chemical analog or simply an analog, is a compound having a structure similar to that of another compound, but differing from it in respect to a certain component.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.
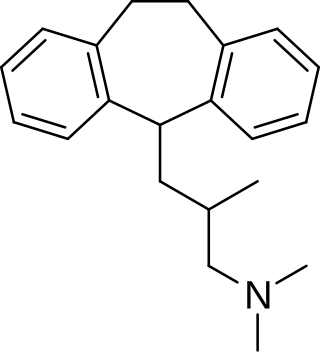
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.
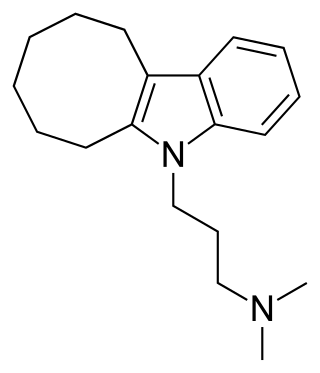
Iprindole, sold under the brand names Prondol, Galatur, and Tertran, is an atypical tricyclic antidepressant (TCA) that has been used in the United Kingdom and Ireland for the treatment of depression but appears to no longer be marketed. It was developed by Wyeth and was marketed in 1967. The drug has been described by some as the first "second-generation" antidepressant to be introduced. However, it was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Tricyclics are cyclic chemical compounds that contain three fused rings of atoms.
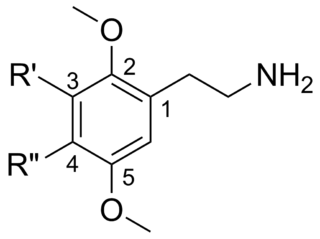
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring. Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds. Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL. Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.

Befuraline (DIV-154) is a psychoactive drug and member of the piperazine chemical class which was developed in Germany in the 1970s. Befuraline has stimulant and antidepressant effects and has seen some use in Germany and France, although it has never become widely used. Befuraline's active metabolite benzylpiperazine is likely to contribute to its effects.

Substituted Methylenedioxyphenethylamines represent a diverse chemical class of compounds derived from phenethylamines. This category encompasses numerous psychoactive substances with entactogenic, psychedelic, and/or stimulant properties, in addition to entheogens. These compounds find application as research chemicals, designer drugs, and recreational substances.

Ariadne is a little-known psychoactive drug. It is a homologue of the psychedelics 2C-D and DOM. Ariadne was first synthesized by Alexander Shulgin. In his 1991 book PiHKAL, Shulgin reported testing Ariadne up to a dose of 32 mg, and reported that it produced "the alert of a psychedelic, with none of the rest of the package". Very little published data exists about the human pharmacology of Ariadne apart from Shulgin's limited testing; unpublished human trials reportedly observed some psychoactive effects, but no hallucinations.

SNC-80 is an opioid analgesic compound that selectively activates μ–δ opioid receptor heteromers and is used in scientific research. It was discovered in 1994.

4-Bromomethcathinone is a psychoactive drug and research chemical of the phenethylamine, amphetamine, and cathinone chemical classes. It acts as a serotonin and norepinephrine reuptake inhibitor, but acts more like an antidepressant than a stimulant. Some 4-halogenated cathinones seem to share the selective serotonergic neurotoxicity of their corresponding amphetamine analogues.

Ciclazindol (WY-23409) is an antidepressant and anorectic drug of the tetracyclic chemical class that was developed in the mid to late 1970s, but was never marketed. It acts as a norepinephrine reuptake inhibitor, and to a lesser extent as a dopamine reuptake inhibitor. Ciclazindol has no effects on the SERT, 5-HT receptors, mACh receptors, or α-adrenergic receptors, and has only weak affinity for the H1 receptor. As suggested by its local anesthetic properties, ciclazindol may also inhibit sodium channels. It is known to block potassium channels as well.
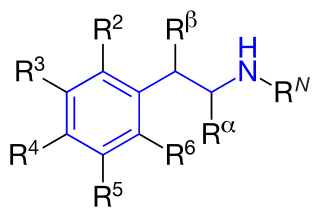
Substituted phenethylamines are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents.
The molecular formula C11H15N (molar mass: 161.24 g/mol) may refer to:

Aptazapine (developmental code name CGS-7525A) is a tetracyclic antidepressant (TeCA) that was assayed in clinical trials for the treatment of depression in the 1980s but was never marketed. It is a potent α2-adrenergic receptor antagonist with ~10x the strength of the related compound mianserin and has also been shown to act as a 5-HT2 receptor antagonist and H1 receptor inverse agonist, while having no significant effects on the reuptake of serotonin or norepinephrine. Based on its pharmacological profile, aptazapine may be classified as a noradrenergic and specific serotonergic antidepressant (NaSSA).

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.



















