
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

Phenylpropanolamine (PPA), sold under many brand names, is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was previously commonly used in prescription and over-the-counter cough and cold preparations. The medication is taken by mouth.

3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
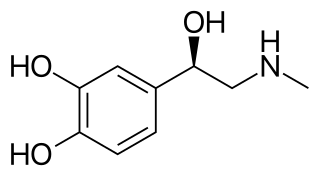
Sympathomimetic drugs are stimulant compounds which mimic the effects of endogenous agonists of the sympathetic nervous system. Examples of sympathomimetic effects include increases in heart rate, force of cardiac contraction, and blood pressure. The primary endogenous agonists of the sympathetic nervous system are the catecholamines, which function as both neurotransmitters and hormones. Sympathomimetic drugs are used to treat cardiac arrest and low blood pressure, or even delay premature labor, among other things.

Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono-substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone.

WIN 35,428 is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
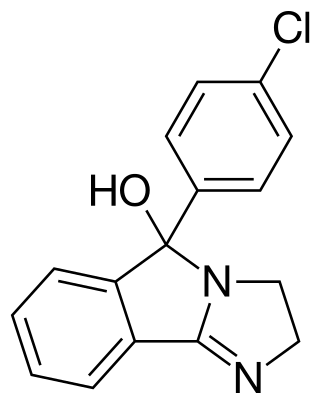
Mazindol is a stimulant drug which is used as an appetite suppressant. It was developed by Sandoz-Wander in the 1960s.
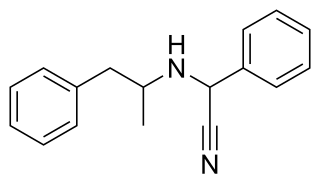
Amfetaminil is a stimulant drug derived from amphetamine, which was developed in the 1970s and used for the treatment of obesity, ADHD, and narcolepsy. It has largely been withdrawn from clinical use following problems with abuse. The drug is a prodrug to amphetamine.

2-MDP (U-23807A) is a dissociative anaesthetic drug which has been found to be an NMDA antagonist and produces similar effects to PCP in animals. The levo or (−)-isomer is the active form of the drug. It also has stimulant effects, having only around one third the potency of amphetamine by weight, but with a long duration of action, lasting more than 24 hours from a single oral dose.

2-Benzylpiperidine is a stimulant drug of the piperidine class. It is similar in structure to other drugs such as methylphenidate and desoxypipradrol but around one twentieth as potent, and while it boosts norepinephrine levels to around the same extent as d-amphetamine, it has very little effect on dopamine levels, with its binding affinity for the dopamine transporter around 175 times lower than for the noradrenaline transporter. 2-benzylpiperidine is little used as a stimulant, with its main use being as a synthetic intermediate in the manufacture of other drugs.
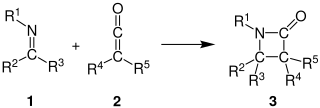
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.
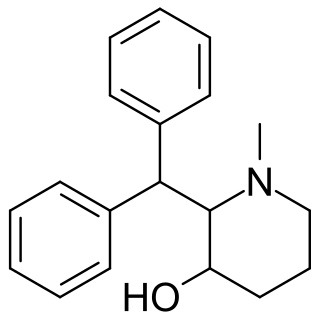
SCH-5472. is a stimulant drug developed by Schering-Plough in the 1950s.

Fenmetozole (DH-524) is a drug which was patented as an antidepressant, but was later studied as an antagonist of the effects of ethanol, though results were poor and it even increased its effects in some cases. It acts as an α2-adrenergic receptor antagonist similarly to other imidazoles like idazoxan. It was never marketed.

G-130 is a drug with stimulant and anorectic effects, related to phenmetrazine.

Quifenadine is a 2nd generation antihistamine drug, marketed mainly in post-Soviet countries. Chemically, it is a quinuclidine derivative.

p-Hydroxynorephedrine (PHN), or 4-hydroxynorephedrine, is the para-hydroxy analog of norephedrine and an active sympathomimetic metabolite of amphetamine in humans. When it occurs as a metabolite of amphetamine, it is produced from both p-hydroxyamphetamine and norephedrine.

4-Hydroxyphenylacetone is the para-hydroxy analog of phenylacetone, an inactive metabolite of amphetamine in humans. When it occurs as a metabolite of amphetamine, it is produced directly from the inactive metabolite phenylacetone.

Substituted β-hydroxyamphetamines, also known as substituted phenylisopropanolamines, substituted phenylpropanolamines, substituted norephedrines, or substituted cathinols, are derivatives of β-hydroxyamphetamine with one or more chemical substituents. They are substituted phenethylamines, phenylethanolamines (β-hydroxyphenethylamines), and amphetamines (α-methylphenethylamines), and are closely related to but distinct from the substituted cathinones (β-ketoamphetamines). Examples of β-hydroxyamphetamines include the β-hydroxyamphetamine stereoisomers phenylpropanolamine and cathine and the stereospecific N-methylated β-hydroxyamphetamine derivatives ephedrine and pseudoephedrine, among many others.





















