
Amides of lysergic acid are collectively known as lysergamides or ergoamides, and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors. Lysergamides contain an embedded tryptamine structure, and as a result can produce similar, often psychedelic, effects to those of the true tryptamines.

1,3-Benzodioxolylbutanamine is an entactogenic drug of the phenethylamine, amphetamine, and phenylisobutylamine families. It is the α-ethyl analog of MDPEA and MDA and the methylenedioxy analogue of α-ethylphenethylamine.

MN 18 is an indazole-based synthetic cannabinoid that is an agonist for the cannabinoid receptors, with Ki values of 45.72 nM at CB1 and 11.098 nM at CB2 and EC50 values of 2.028 nM at CB1 and 1.233 nM at CB2, and has been sold online as a designer drug. It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen. MN-18 metabolism has been described in literature.

4'-Methyl-α-pyrrolidinopropiophenone is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s, along with a number of other pyrrolidinophenone derivatives. Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends such as "NRG-3".

Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

Pentylone is a stimulant developed in the 1960s. It is a substituted cathinone that has been identified in some samples of powders sold as "NRG-1", along with varying blends of other cathinone derivatives including flephedrone, MDPBP, MDPV, and 4-MePPP. It was also found in combination with 4-MePPP being sold as "NRG-3". Reports indicate side effects include paranoia, agitation, and insomnia, with effects lasting for several days at high doses.
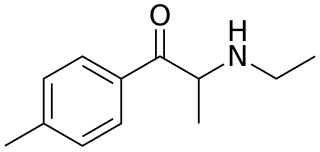
4-Methylethcathinone or 4-MEC is a chemical that bears a chemical resemblance to mephedrone. Due to its similarity to mephedrone, it is thought to be a stimulant and entactogen drug of the phenethylamine, amphetamine, and cathinone chemical classes. It has been marketed alone or in mixtures with other substituted cathinones under the name "NRG-2", although other blends such as "NRG-1" may have been more ambiguous with their ingredients.

AB-FUBINACA (AMB-FUBINACA) is a psychoactive drug that acts as a potent agonist for the cannabinoid receptors, with Ki values of 0.9 nM at CB1 and 23.2 nM at CB2 and EC50 values of 1.8 nM at CB1 and 3.2 nM at CB2. It was originally developed by Pfizer in 2009 as an analgesic medication but was never pursued for human use. In 2012, it was discovered as an ingredient in synthetic cannabinoid blends in Japan, along with a related compound AB-PINACA, which had not previously been reported.
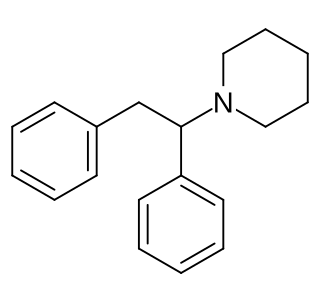
Diphenidine is a dissociative anesthetic that has been sold as a designer drug. The synthesis of diphenidine was first reported in 1924, and employed a Bruylants reaction analogous to the one that would later be used to discover phencyclidine in 1956. Shortly after the 2013 UK ban on arylcyclohexylamines, diphenidine and the related compound methoxphenidine became available on the grey market. Anecdotal reports describe high doses of diphenidine producing "bizarre somatosensory phenomena and transient anterograde amnesia." Diphenidine and related diarylethylamines have been studied in vitro as treatments for neurotoxic injury and are antagonists of the NMDA receptor. In dogs diphenidine exhibits greater antitussive potency than codeine phosphate.

βk-2C-B (βeta-keto-4-bromo-2,5-dimethoxyphenylamine), also known as bk-2C-B, is a novel psychedelic substance. It is the beta (β) ketone structural analogue of 2C-B, a psychedelic drug of the 2C family. It is used as a recreational drug, usually taken orally. βk-2C-B is a controlled substance in Canada, Germany, Switzerland, and the United Kingdom.

Flubromazepam is a benzodiazepine derivative which was first synthesized in 1960, but was never marketed and did not receive any further attention or study until late 2012 when it appeared on the grey market as a novel designer drug.

3-Methoxyeticyclidine (3-MeO-PCE), also known as methoxieticyclidine, is a dissociative anesthetic that is qualitatively similar to PCE and PCP and has been sold online as a designer drug.

The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached, and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name Benzofury.
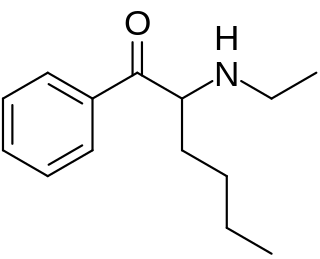
N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.
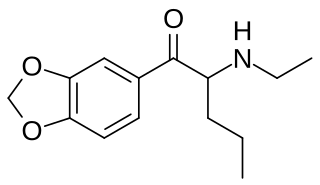
N-Ethylpentylone is a substituted cathinone and stimulant drug which was developed in the 1960s.

α-Pyrrolidinoisohexanophenone is a stimulant drug of the cathinone class that has been sold online as a designer drug. It acts as a potent norepinephrine-dopamine reuptake inhibitor (NDRI). In July 2016 α-PHiP was first identified as a designer drug when it was reported to the EMCDDA by a forensic laboratory in Slovenia. It is a positional isomer of pyrovalerone, with the methyl group shifted from the 4-position of the aromatic ring to the 4-position of the acyl chain. Similarly to other cathinones, use of α-PiHP can result in compulsive redosing, addiction, anxiety, paranoia, and psychosis.

MDMB-4en-PINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. MDMB-4en-PINACA was first identified in Europe in 2017. In 2021, MDMB-4en-PINACA was the most common synthetic cannabinoid identified by the Drug Enforcement Administration in the United States. MDMB-4en-PINACA differs from 5F-MDMB-PINACA due to replacement of 5-fluoropentyl with a pent-4-ene moiety (4-en).

ADB-BINACA (also known as ADMB-BZINACA using EMCDDA naming standards) is a cannabinoid designer drug that has been found as an ingredient in some synthetic cannabis products. It was originally developed by Pfizer as a potential analgesic, and is a potent agonist of the CB1 receptor with a binding affinity (Ki) of 0.33 nM and an EC50 of 14.7 nM.
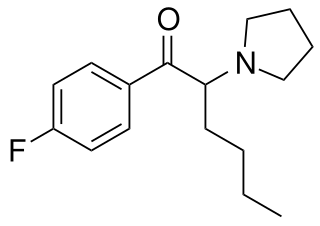
4-Fluoro-alpha-PHP (4F-PHP) is a recreational designer drug from the substituted cathinone family with stimulant effects, which first appeared on the illicit market in around 2017.



















