
Monoamine transporters (MATs) are proteins that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. The three major classes are serotonin transporters (SERTs), dopamine transporters (DATs), and norepinephrine transporters (NETs) and are responsible for the reuptake of their associated amine neurotransmitters. MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and post-translational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylphenidate (Ritalin) and amphetamine in its many forms and derivatives methamphetamine (Desoxyn) and lisdexamfetamine (Vyvanse). Furthermore, drugs such as MDMA and natural alkaloids such as cocaine exert their effects in part by their interaction with MATs, by blocking the transporters from mopping up dopamine, serotonin, and other neurotransmitters from the synapse.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and migraine prevention. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.

Nomifensine, sold under the brand names Merital and Alival, is a norepinephrine–dopamine reuptake inhibitor (NDRI), i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters. This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.

Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.

Indatraline hydrochloride is an antidepressive agent and non-selective monoamine transporter inhibitor that blocks the reuptake of dopamine, norepinephrine, and serotonin with similar efficacy to cocaine. This compound may be used to treat cocaine addictions as its effects have a slower onset and a longer duration than those of cocaine. Lu 19-005 has been shown to block the action of methamphetamine and MDMA in laboratory experiments.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

RTI-126 is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter. RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.
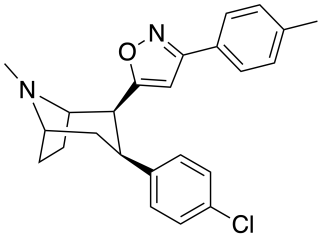
RTI(-4229)-336, is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine, however it produces relatively mild stimulant effects, with a slow onset and long duration of action. These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse. RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration, and significantly reduces rates of cocaine use, especially when combined with SSRIs, and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.
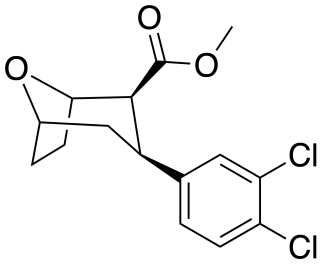
Tropoxane (O-1072) is an aryloxytropane derivative drug developed by Organix Inc., which acts as a stimulant and potent dopamine and serotonin reuptake inhibitor. It is an analogue of dichloropane where the amine nitrogen has been replaced by an oxygen ether link, demonstrating that the amine nitrogen is not required for DAT binding and reuptake inhibition.
A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain. No selective and robust DRAs are currently known. On the other hand, many releasing agents of both dopamine and norepinephrine and of serotonin, norepinephrine, and dopamine are known. Serotonin–dopamine releasing agents (SDRAs), for instance 5-chloro-αMT, are much more rare and are not selective for dopamine release but have also been developed. Examples of major NDRAs include the psychostimulants amphetamine and methamphetamine, while an example of an SNDRA is the entactogen methylenedioxymethamphetamine (MDMA). These drugs are frequently used for recreational purposes and encountered as drugs of abuse. Selective DRAs, as well as NDRAs, have medical applications in the treatment of attention deficit hyperactivity disorder (ADHD).

3-Fluoroamphetamine is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin. It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.
EXP-561 is an investigational drug that acts as an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. It was developed in the 1960s by Du Pont and was suggested as a potential antidepressant but failed in trials and was never marketed.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

4-Fluoromethylphenidate is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.

O-2390 is a recreational designer drug from the substituted cathinone family, which acts as a potent inhibitor of dopamine and noradrenaline reuptake in vitro, with weaker but still significant inhibition of serotonin reuptake.















