
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.
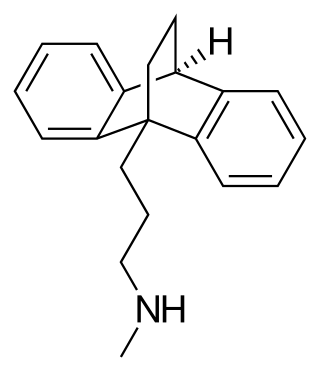
Maprotiline, sold under the brand name Ludiomil among others, is a tetracyclic antidepressant (TeCA) that is used in the treatment of depression. It may alternatively be classified as a tricyclic antidepressant (TCA), specifically a secondary amine. In terms of its chemistry and pharmacology, maprotiline is closely related to such-other secondary-amine TCAs as nortriptyline and protriptyline and has similar effects to them, albeit with more distinct anxiolytic effects. Additionally, whereas protriptyline tends to be somewhat more stimulating and in any case is distinctly more-or-less non-sedating, mild degrees of sedation may be experienced with maprotiline.

Amitriptyline, sold under the brand name Elavil among others, is a tricyclic antidepressant primarily used to treat major depressive disorder, a variety of pain syndromes such as neuropathic pain, fibromyalgia, migraine and tension headaches. Due to the frequency and prominence of side effects, amitriptyline is generally considered a second-line therapy for these indications.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and migraine prevention. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

Amoxapine, sold under the brand name Asendin among others, is a tricyclic antidepressant (TCA). It is the N-demethylated metabolite of loxapine. Amoxapine first received marketing approval in the United States in 1980, approximately 10 to 20 years after most of the other TCAs were introduced in the United States.

Imipramine, sold under the brand name Tofranil, among others, is a tricyclic antidepressant (TCA) mainly used in the treatment of depression. It is also effective in treating anxiety and panic disorder. Imipramine is taken by mouth.

Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.

Clomipramine, sold under the brand name Anafranil among others, is a tricyclic antidepressant (TCA). It is used in the treatment of various conditions, most-notably obsessive–compulsive disorder but also many other disorders, including panic disorder, major depressive disorder, trichotilomania, body dysmorphic disorder and chronic pain. It has also been notably used to treat premature ejaculation and the cataplexy associated with narcolepsy.

Nortriptyline, sold under the brand name Pamelor, among others, is a medication used to treat depression. This medicine is also sometimes used for neuropathic pain, attention deficit hyperactivity disorder (ADHD), smoking cessation and anxiety. As with many antidepressants, its use for young people with depression and other psychiatric disorders may be limited due to increased suicidality in the 18–24 population initiating treatment. Nortriptyline is a less preferred treatment for ADHD and stopping smoking. It is taken by mouth.

Doxepin is a medication belonging to the tricyclic antidepressant (TCA) class of drugs used to treat major depressive disorder, anxiety disorders, chronic hives, and insomnia. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs, however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression. Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs. It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.
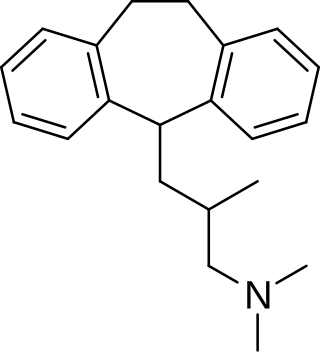
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Lofepramine, sold under the brand names Gamanil, Lomont, and Tymelyt among others, is a tricyclic antidepressant (TCA) which is used to treat depression. The TCAs are so named as they share the common property of having three rings in their chemical structure. Like most TCAs lofepramine is believed to work in relieving depression by increasing concentrations of the neurotransmitters norepinephrine and serotonin in the synapse, by inhibiting their reuptake. It is usually considered a third-generation TCA, as unlike the first- and second-generation TCAs it is relatively safe in overdose and has milder and less frequent side effects.
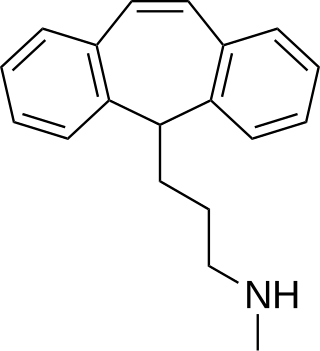
Protriptyline, sold under the brand name Vivactil among others, is a tricyclic antidepressant (TCA), specifically a secondary amine, indicated for the treatment of depression and attention-deficit hyperactivity disorder (ADHD). Uniquely among most of the TCAs, protriptyline tends to be energizing instead of sedating, and is sometimes used for narcolepsy to achieve a wakefulness-promoting effect.
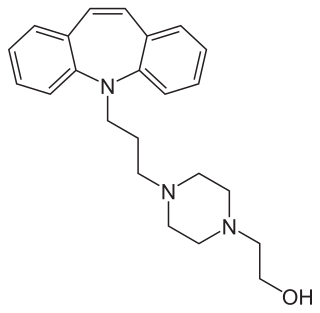
Opipramol, sold under the brand name Insidon among others, is an anxiolytic and tricyclic antidepressant that is used throughout Europe. Despite chemically being a tricyclic dibenzazepine (iminostilbene) derivative similar to imipramine, opipramol is not a monoamine reuptake inhibitor like most other tricyclic antidepressants, and instead, uniquely among antidepressants, acts primarily as a SIGMAR1 agonist. It was developed by Schindler and Blattner in 1961.

Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.
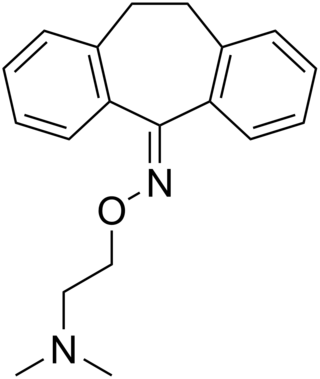
Noxiptiline, also known as noxiptyline and dibenzoxine, is a tricyclic antidepressant (TCA) that was introduced in Europe in the 1970s for the treatment of depression. It has imipramine-like effects, acting as a serotonin and norepinephrine reuptake inhibitor, among other properties. Of the TCAs, noxiptiline has been described as one of the most effective, rivaling amitriptyline in clinical efficacy.

Amitriptylinoxide, or amitriptyline N-oxide, is a tricyclic antidepressant (TCA) which was introduced in Europe in the 1970s for the treatment of depression.
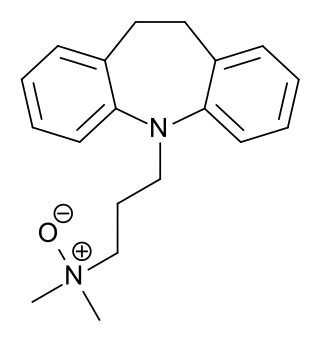
Imipraminoxide, or imipramine N-oxide, is a tricyclic antidepressant (TCA) that was introduced in Europe in the 1960s for the treatment of depression.




















