Proton-pump inhibitors (PPIs) are a class of medications that cause a profound and prolonged reduction of stomach acid production. They do so by irreversibly inhibiting the stomach's H+/K+ ATPase proton pump.

H2 antagonists, sometimes referred to as H2RAs and also called H2 blockers, are a class of medications that block the action of histamine at the histamine H2 receptors of the parietal cells in the stomach. This decreases the production of stomach acid. H2 antagonists can be used in the treatment of dyspepsia, peptic ulcers and gastroesophageal reflux disease. They have been surpassed by proton pump inhibitors (PPIs). The PPI omeprazole was found to be more effective at both healing and alleviating symptoms of ulcers and reflux oesophagitis than the H2 blockers ranitidine and cimetidine.

Zollinger–Ellison syndrome is rare disease in which tumors cause the stomach to produce too much acid, resulting in peptic ulcers. Symptoms include abdominal pain and diarrhea.
The histamine receptors are a class of G protein–coupled receptors which bind histamine as their primary endogenous ligand.
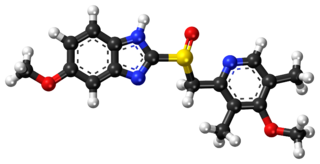
Esomeprazole, sold under the brand name Nexium [or Neksium] among others, is a medication which reduces stomach acid. It is used to treat gastroesophageal reflux disease, peptic ulcer disease, and Zollinger–Ellison syndrome. Its effectiveness is similar to that of other proton pump inhibitors (PPIs). It is taken by mouth or injection into a vein.

Rabeprazole, sold under the brand name Aciphex, among others, is a medication that decreases stomach acid. It is used to treat peptic ulcer disease, gastroesophageal reflux disease, and excess stomach acid production such as in Zollinger–Ellison syndrome. It may also be used in combination with other medications to treat Helicobacter pylori. Effectiveness is similar to other proton pump inhibitors (PPIs). It is taken by mouth.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.

Ranitidine, sold under the brand name Zantac among others, is a medication used to decrease stomach acid production. It is commonly used in treatment of peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome. It can be given by mouth, injection into a muscle, or injection into a vein. In September 2019, the probable carcinogen N-nitrosodimethylamine (NDMA) was discovered in ranitidine products from a number of manufacturers, resulting in recalls.

Nizatidine is a histamine H2 receptor antagonist that inhibits stomach acid production, and is commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease.

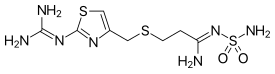
Metiamide is a histamine H2 receptor antagonist developed from another H2 antagonist, burimamide. It was an intermediate compound in the development of the successful anti-ulcer drug cimetidine (Tagamet).

A vagotomy is a surgical procedure that involves removing part of the vagus nerve. It is performed in the abdomen.

Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic drug that can be bought without a prescription and provides relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects. Antihistamines are usually for short-term treatment. Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection. Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.
Lafutidine (INN) is a second generation histamine H2 receptor antagonist having multimodal mechanism of action and used to treat gastrointestinal disorders. It is marketed in South Korea, Japan and India.

Troxipide is a drug used in the treatment of gastroesophageal reflux disease. Troxipide is a systemic non-antisecretory gastric cytoprotective agent with anti-ulcer, anti-inflammatory and mucus secreting properties irrespective of pH of stomach or duodenum. Troxipide is currently marketed in Japan (Aplace), China (Shuqi), South Korea (Defensa), and India (Troxip). It is used for the management of gastric ulcers, and amelioration of gastric mucosal lesions in acute gastritis and acute exacerbation of chronic gastritis.
Ebrotidine is an H2 receptor antagonist with gastroprotective activity against ethanol-, aspirin- or stress-induced gastric mucosal damage. The antisecretory properties of ebrotidine are similar to those of ranitidine, and approximately 10-fold greater than those of cimetidine. Ebrotidine has anti-Helicobacter pylori activity via inhibition of the urease enzyme and the proteolytic and mucolytic activities of the bacterium. However, its activity is synergistic with a number of antibacterial agents. Ebrotidine counteracts the inhibitory effects of H. pylori lipopolysaccharides. Ebrotidine was withdrawn from the market due to risks of hepatotoxicity.
There are several classes of drugs for acid-related disorders, such as dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD), or laryngopharyngeal reflux.
Equine gastric ulcer syndrome (EGUS) is a common cause of colic and decreased performance in horses. Horses form ulcers in the mucosa of the stomach, leading to pain, decreased appetite, weight loss, and behavioral changes. Treatment generally involves reducing acid production of the stomach and dietary management. Unlike some animals, however, stomach rupture is rare, and the main goal of treating is to reduce pain and improve performance of animals used for showing or racing.
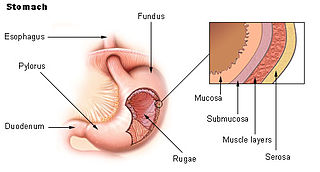
Acid peptic diseases, such as peptic ulcers, Zollinger-Ellison syndrome, and gastroesophageal reflux disease, are caused by distinct but overlapping pathogenic mechanisms involving acid effects on mucosal defense. Acid reflux damages the esophageal mucosa and may also cause laryngeal tissue injury, leading to the development of pulmonary symptoms.
Ranitidine bismuth citrate - drug, which has antisecretory and bactericidal action.
Ibuprofen/famotidine, sold under the brand name Duexis, is a fixed-dose combination medication used for the treatment of rheumatoid arthritis and osteoarthritis. It contains ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID) and famotidine, a histamine H2-receptor antagonist.