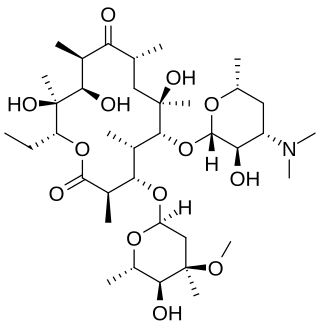
Erythromycin is an antibiotic used for the treatment of a number of bacterial infections. This includes respiratory tract infections, skin infections, chlamydia infections, pelvic inflammatory disease, and syphilis. It may also be used during pregnancy to prevent Group B streptococcal infection in the newborn, and to improve delayed stomach emptying. It can be given intravenously and by mouth. An eye ointment is routinely recommended after delivery to prevent eye infections in the newborn.

Clarithromycin, sold under the brand name Biaxin among others, is an antibiotic used to treat various bacterial infections. This includes strep throat, pneumonia, skin infections, H. pylori infection, and Lyme disease, among others. Clarithromycin can be taken by mouth as a tablet or liquid or can be infused intravenously.
H1 antagonists, also called H1 blockers, are a class of medications that block the action of histamine at the H1 receptor, helping to relieve allergic reactions. Agents where the main therapeutic effect is mediated by negative modulation of histamine receptors are termed antihistamines; other agents may have antihistaminergic action but are not true antihistamines.

Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis and hives. It is also available in drug combinations such as loratadine/pseudoephedrine, in which it is combined with pseudoephedrine, a nasal decongestant. It is taken orally.

Brompheniramine, sold under the brand name Dimetapp among others, is a first-generation antihistamine drug of the propylamine (alkylamine) class. It is indicated for the treatment of the symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing. Like the other first-generation drugs of its class, it is considered a sedating antihistamine.
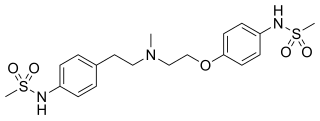
Dofetilide is a class III antiarrhythmic agent. It is marketed under the trade name Tikosyn by Pfizer, and is available in the United States in capsules containing 125, 250, and 500 μg of dofetilide. It is not available in Europe or Australia.
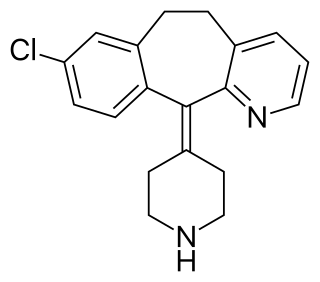
Desloratadine. sold under the brand name Clarinex among others, is a tricyclic H1 inverse agonist that is used to treat allergies. It is an active metabolite of loratadine.

Cetirizine is a second-generation antihistamine used to treat allergic rhinitis, dermatitis, and urticaria (hives). It is taken by mouth. Effects generally begin within thirty minutes and last for about a day. The degree of benefit is similar to other antihistamines such as diphenhydramine, which is a first-generation antihistamine.
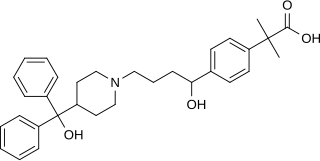
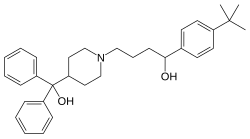
Fexofenadine, sold under the brand name Allegra among others, is an antihistamine pharmaceutical drug used in the treatment of allergy symptoms, such as hay fever and urticaria.
A prodrug is a pharmacologically inactive medication or compound that, after intake, is metabolized into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug is absorbed, distributed, metabolized, and excreted (ADME).

Procainamide (PCA) is a medication of the antiarrhythmic class used for the treatment of cardiac arrhythmias. It is a sodium channel blocker of cardiomyocytes; thus it is classified by the Vaughan Williams classification system as class Ia. In addition to blocking the INa current, it inhibits the IKr rectifier K+ current. Procainamide is also known to induce a voltage-dependent open channel block on the batrachotoxin (BTX)-activated sodium channels in cardiomyocytes.

Levocetirizine, sold under the brand name Xyzal, among others, is a second-generation antihistamine used for the treatment of allergic rhinitis and long-term hives of unclear cause. It is less sedating than older antihistamines. It is taken by mouth.
Azelastine, sold under the brand name Astelin among others, is a H1 receptor-blocking medication primarily used as a nasal spray to treat allergic rhinitis (hay fever) and as eye drops for allergic conjunctivitis. Other uses may include asthma and skin rashes for which it is taken by mouth. Onset of effects is within minutes when used in the eyes and within an hour when used in the nose. Effects last for up to 12 hours.

Acrivastine is a medication used for the treatment of allergies and hay fever. It is a second-generation H1-receptor antagonist antihistamine and works by blocking histamine H1 receptors.

Carbinoxamine is an antihistamine and anticholinergic agent. It is used for hay fever, vasomotor rhinitis, mild urticaria, angioedema, dermatographism and allergic conjunctivitis. Carbinoxamine is a histamine antagonist, specifically an H1-antagonist. The maleic acid salt of the levorotatory isomer is sold as the prescription drug rotoxamine.

Azatadine (Optimine) is a first-generation antihistamine and anticholinergic drug that was synthesized in 1963 by Schering-Plough, a former American pharmaceutical company.

Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic drug that can be bought without a prescription and provides relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects. Antihistamines are usually for short-term treatment. Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection. Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.
Raymond L. Woosley is an American pharmacologist who is the founding president and chairman of the board for AZCERT, a not-for-profit organization dedicated to improved outcomes from the use of medications. Prior to leading AZCERT, he was founder and President of Critical Path Institute (C-Path). C-Path is an independent, non-profit organization created by the U.S. Food and Drug Administration (FDA) and the University of Arizona to help launch the critical path initiative. Previously, he has served as Vice-President for Health Sciences and Dean of the College of Medicine at the University of Arizona. He is Professor of Medicine and Biomedical Informatics in the University of Arizona College of Medicine - Phoenix, Arizona.
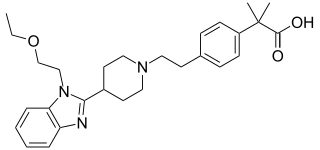
Bilastine is an antihistamine medication used to treat hives (urticaria), allergic rhinitis and itchy inflamed eyes (allergic conjunctivitis) caused by an allergy. It is a second-generation antihistamine and takes effect by selectively inhibiting the histamine H1 receptor, preventing these allergic reactions. Bilastine has an effectiveness similar to cetirizine, fexofenadine, and desloratadine.
Fexofenadine/pseudoephedrine, sold under the brand name Allegra-D among others, is a fixed-dose combination medication used for the treatment of nasal congestion and other symptoms of allergies and the common cold. It contains fexofenadine, as the hydrochloride, an antihistamine; and pseudoephedrine, as the hydrochloride, a nasal decongestant.